 Category: IPEC Federation
Category: IPEC FederationWelcome to our first 2024 issue of the IPEC Federation bulletin, which we hope you will find interesting. In this edition, we delve into the priority objectives for 2024 and regional updates.
Read more Category: IPEC Federation
Category: IPEC FederationThe Medicine Maker hosted an interview with Dr. Frank Milek to highlight the importance of ensuring security of the excipient supply chain in light of the recent incidents caused by DEG contamination in different areas of the world.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the updated IPEC Position Paper on “The Role of Excipients in Determining N-Nitrosamine Risks for Drug Products”.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised and updated IPEC Quality Agreement Guide and Template(s) for Pharmaceutical Excipients.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised and updated IPEC TUPP Guide for Pharmaceutical Excipients.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised and updated IPEC General Glossary of Terms and Acronyms for Pharmaceutical Excipients.
Read more Category: IPEC Europe
Category: IPEC EuropeAre you looking for an internship in administrative and event support? Join a dynamic not-for-profit association representing pharmaceutical excipients!
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of an updated position paper, “Latest fatal incidents with contaminated medicinal syrup during 2022/2023”.
Read more Category: IPEC Federation
Category: IPEC FederationWelcome to our first 2023 issue of the IPEC Federation bulletin, which we hope you will find interesting. In this edition, we delve into the priority objectives for 2023 and regional updates.
Read more Category: IPEC Federation
Category: IPEC Federation IPEC Federation announces the availability of the new Excipient Information Package User Guide and Template, Part IV: Sustainability.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the updated IPEC Significant Change Guide for Pharmaceutical Excipients (Version 5, 2023)
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of its “Questionnaire for Excipient Nitrosamines Risk Evaluation” (version 1).
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the updated IPEC Stability Guide for Pharmaceutical Excipients (Version 2, 2022)
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the updated IPEC Certificate of Analysis Guide for Pharmaceutical Excipients (Version 2, 2022).
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation and the Pharmaceutical Quality Group (PQG) are pleased to announce the availability of the revised IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients (version 5).
Read more Category: IPEC Europe
Category: IPEC EuropePlease complete a quick survey on Certificates of Suitability by 11 May 2022.
Read more Category: IPEC Federation
Category: IPEC FederationWelcome to the latest edition of the IPEC Federation bulletin. In this edition, we delve into the Federation’s objectives for 2022 and the recent meeting with PDG.
Read more Category: IPEC Federation
Category: IPEC FederationThis position paper describes IPEC Federation’s position on the role of excipients when conducting N-nitrosamine (nitrosamine) risk assessments for drug products
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Europe is pleased to announce the availability of a Best Practices document, “Executing a Multicompendial Compliance Strategy for Pharmaceutical Excipients”
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the new IPEC Safety Guide for Pharmaceutical Excipients.
Read more Category: Stakeholders
Category: StakeholdersAn interesting article sets the key theme that will shape the excipient landscape in the near future such as regulations, innovation, economics and sustainability.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised IPEC Good Distribution Practices Audit Guide for Pharmaceutical Excipients (Version 3, 2021).
Read more Category: IPEC Federation
Category: IPEC FederationWelcome to the latest edition of the IPEC Federation bulletin, which we hope you will find interesting. In this edition, we delve into the Federation’s objectives for 2021 and news from the IPECs around the world.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the Spanish version of the IPEC Good Distribution Practices Guide for Pharmaceutical Excipients (version 2, 2017).
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Europe and EFCG continue to make the case that the importance of novel excipients needs to be fully recognised and are pleased to see that others are beginning to take notice too.
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Europe announces the availability of its revised “Questionnaire for Excipient Nitrosamines Risk Evaluation” (version 3).
Read more Category: IPEC Europe
Category: IPEC EuropeNovel excipients are essential for the development of new medicines and vaccines. Read the joint IPEC Europe-EFCG position paper.
Read more Category: IPEC Federation
Category: IPEC FederationThis position paper describes IPEC Federation’s position on the risk classification of lactose in all oral preparations.
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Federation announces the availability of the updated IPEC Glossary.
To consult it online, click here
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the new International Pharmaceutical Excipients Council Validation Guide for Pharmaceutical Excipients.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised IPEC Qualification of Excipients for Use in Pharmaceuticals Guide and Checklist.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the new International Pharmaceutical Excipients Council GMP Certification Scheme and Certification Body Qualification Guide for Pharmaceutical Excipients.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of a new guide: Incorporation of Pharmaceutical Excipients into Product Development using Quality-by-Design (QbD guide).
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Europe, EFCG and APIC address the complex pharmaceutical supply chain in Europe in a joint infographic.
Read more Category: IPEC Europe
Category: IPEC EuropeAre you a drug formulator? Help us build the case for novel excipients in Europe and complete the survey by 31 August!
Read more Category: IPEC Federation
Category: IPEC FederationA position paper that explains the various excipient GMP guides and standards currently available has been published by the IPEC Federation.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of a new position paper on Data Integrity for Pharmaceutical Grade Excipients.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation is pleased to announce the revised IPEC Excipient Composition Guide (Version 2, 2020).
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation announces the availability of the revised IPEC Excipient Information Package - User Guide and Templates (EIP) (Version 4, 2020).
Read more Category: EXCiPACT
Category: EXCiPACTEXCiPACT issued a new procedure for audit postponement and the use of remote audits of pharmaceutical excipients suppliers.
Read more Category: IPEC Federation
Category: IPEC FederationWelcome to the first edition of the IPEC Federation Connect, a bulletin providing a periodic round-up of what the IPEC Federation and other IPECs have been up to!
Read more Category: IPEC Federation
Category: IPEC FederationPDA and IPEC Federation announce the joint publication of Technical Report No. 54-6 - Formalized Risk Assessment for Excipients.
Read more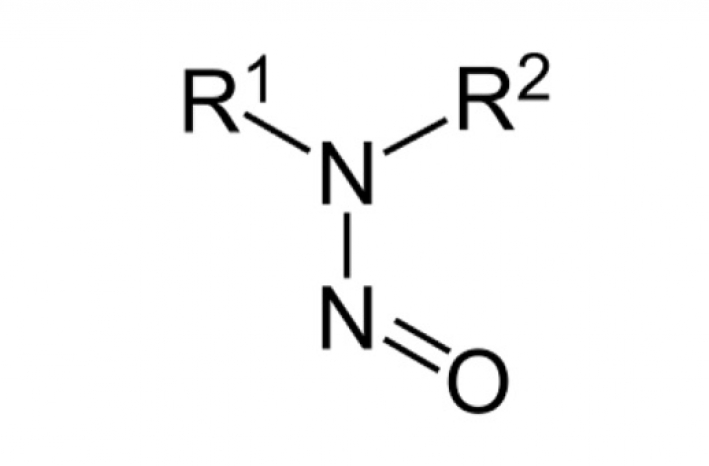 Category: IPEC Europe
Category: IPEC EuropeIPEC Europe developed a questionnaire in consultation with other pharmaceutical-related associations (including EFPIA) to facilitate gathering information on pharmaceutical excipients for drug product manufacturers to perform nitrosamine risk evaluations.
Read more Category: IPEC Federation
Category: IPEC FederationIPEC Federation publishes its position paper on supply chain security for Pharmaceutical Grade Excipients.
Read more Category: IPEC Europe
Category: IPEC EuropeIPEC Europe released a position paper and a Stimuli article to highlight how the absence of an Excipient Master File system in Europe is a barrier to the introduction of novel excipients and to innovation in the healthcare sector.
Read more 












































