Editorial

Dear readers,
It’s hard to believe that we’re issuing our June publication already and we find ourselves heading for the summer break. And of course, we’re now preparing for those mid-year reviews to measure progress to ensure we can meet our goals by the end of the year. Not only that but September is not far away when the Board will convene with an eye to 2019. We’ll be revisiting our Agenda 2020 to confirm our strategic direction and that we’re working on the right things to get there. More on this in future issues.
Meanwhile, our committees are getting on with things including the IPEC Europe Pharmacopoeial Review & Harmonisation Committee (PR & HC). This group has met twice this year and is developing a structure which will enable us to provide a better forum for important pharmacopoeial issues. We now have a core team in place but we would still like more volunteers who can actively participate in executing its objectives, not least in designing a new approach to excipient harmonisation, more of which later. So once again, let me make a call for any volunteers from our members to get in touch with the secretariat. Thank you for listening!
On the subject of harmonisation, in July, the IPEC Federation will convene for a face-to-face meeting in Beijing, around the ExcipientFest Asia programme of events. An important part of that will be a discussion on how the IPEC Federation intends to move forward its harmonisation agenda which in turn will help to direct IPEC Europe’s position. We’re also hoping that at the same time, we can have discussions with some key stakeholders on international developments within PDG and other pharmacopoeias, where global harmonisation appears to have faltered. We see more and more collaborations between pharmacopoeias bilaterally, note the recent intentions to cooperate between EDQM and India and China Pharmacopoeias. We, as part of the IPEC Federation, need to understand how we can dovetail into those efforts as industry collaboration is pivotal to making a success of harmonisation efforts. For sure in our July/August issue we’ll be reporting back on how these meetings went. And on the horizon there are meeting tentatively planned with PDG and EDQM so we need to get our thoughts together before then.
The summer months are often referred to as the ‘crazy season’ when little seems to get done but there are still some burning issues out there which we need to follow. As members will know, the discussion in France related to the use of Titanium Dioxide in food continues. And of course, China has published its requirements for Registration Documentation for Pharmaceutical Excipients and Excipient Nomenclature. While IPEC Europe’s QRAC and PR&HC teams will be working diligently through these requirements, despite the temptations of sun and sand, of course, any member is welcome to submit its comments to the Secretariat for compositing with other remarks and those of other PECs.
As news items are often in short supply in July and August, we’ll be distributing a combined issue for those months but for any items of major significance, we will issue an alert to our members.
So for now, bonnes vacances to you all!
Frithjof Holtz
IPEC Europe chair
IPEC Europe flies the flag at Making Pharma

IPEC Europe was present at two back-to-back Making Pharma events in the last few weeks – an inaugural event in Brussels, Belgium, and the established exhibition and conference in Coventry, UK – which provided a great opportunity to give the association’s perspective on three important topics.
The presentations across the two events provided an opportunity to make use of IPEC Europe’s new informative video to introduce the association, while IPEC Europe representatives Astrid Stockrahm-Uhling of DFE Pharma, Iain Moore of Croda International, Liz Meehan of AstraZeneca and Christian Becker of BASF gave presentations covering the new Quality Agreement Guide and templates, excipient functionality-related characteristics (FRCs), and novel excipients.
The audience was engaged and inquisitive at both events, with plenty of questions during the sessions and follow-up at IPEC Europe’s stand.
Tackling the topic of excipient FRCs Meehan told delegates that excipients are increasingly being recognised for the critical role they play in pharmaceutical products and – far from being ‘inactive’ - all have functionality that can impact manufacturability, bioavailability, stability and sterility, for example. For some that functionality is more critical than others, and in general there is an increased focus on excipient functionality with regard to technical, regulatory and quality perspectives.
“Understanding excipient functionality is important during drug product design and development, presenting regulatory control strategies and ensuring appropriate GMP for excipients,” she told delegates. “Success relies heavily on close collaboration between excipient suppliers and users, a core philosophy of IPEC Europe.”
At the Brussels conference, Stockrahm-Uhling introduced the new IPEC Federation Quality Agreement Guide and Templates, published last year to replace the earlier 2009 editions, while Moore took on that role at the event in Coventry.
Both explained that the purpose is to set out all the quality management system requirements that must be met by either the supplier or customer to ensure the listed excipient is manufactured “in conformance with regulatory requirements or customer expectations.”
The templates are there to detail the quality activities and responsibilities that should be included in a Quality Agreement that is appropriate for excipients, they said. A good agreement should define all the quality requirements, define which party is responsible for them and detail how any disputes are to be resolved.
“The intent of this (Quality Agreement) is not to ‘rewrite’ GMP requirements,” they stressed.
Finally, Becker took on the topic of novel excipients. He explained that there is a need for new excipients that can help formulate an increasing number of poorly-soluble or permeable active pharmaceutical ingredients (APIs), facilitate new formulation technologies and improve drug stability in hot climates, as well as solve specific functions such as taste-masking to improve compliance.
Becker described the paths available to developers trying to bring novel excipients to market, which in Europe are limited to submitting data on the excipient as part of the marketing authorization holder (MAH) submission for a pharmaceutical product. He pointed out that unlike the US and Japan, Europe lacks a master file system open to excipients. IPEC Europe is continuously advocating the need for an excipient master file system for the EU as this would allow confidential CMC details to be submitted directly to the regulatory authority, rather than to “a possibly large number of applicants for inclusion into the MAH dossier.
Summing up the events. Moore told Excipients Insight that “in my opinion Making Pharma is a great showcase for IPEC Europe activities. There does seem to be more excipient suppliers and users at the event each year.”
That view was also shared by Meehan. “The UK event showed clear evidence that there are increasing numbers of exhibitors with an interest in excipients, both suppliers and users, and it is hoped that the Europe event will follow with that level of success in future,” she said.
IPEC Europe welcomes BMS as new member
IPEC Europe is delighted to welcome Bristol-Myers Squibb as a new member company this month.
The company says its medicines are helping millions of patients around the world in disease areas such as oncology, cardiovascular, immunoscience, fibrosis and others. It has built a pipeline of potential therapies, and is leveraging translational medicine and data analytics to understand how it can deliver the right medicine to the right patient at the right time to achieve the best outcome.
Through the Bristol-Myers Squibb Foundation, it also promotes health equity and seek to improve health outcomes of populations disproportionately affected by serious diseases and conditions, giving new hope to some of the world’s most vulnerable people. Each day, BMS' employees around the world work together for patients.
IPEC Europe calendar

|
| Group |
Q3/4 2018 |
| IPEC Europe Board |
12 September, 27 November |
| GDP Committee |
28-29 August (Brussels), 29-30 October (Germany) |
| Pharmacopoeial Review & Harmonisation Committee |
TBC |
| Quality/Regulatory Affairs Committee |
26 September (telecon), 28 November |
|
EuPFI seeks excipient sponsors for STEP database

The European Paediatric Formulation Initiative (EuPFI) has launched a drive to accelerate the creation of its STEP database, a resource that is used to improve knowledge about excipient safety in children.
STEP (Safety & Toxicity of Excipients for Paediatrics) was set up in 2014 and the number of substances in the database has been growing steadily, but EuPFI wants to speed up the process – and is seeking organisations who are prepared to contribute by sponsoring the inclusion of new excipients.
“Funding and sponsorship can help to deliver information on the safety and toxicity of excipients to facilitate paediatric drug development, by financing excipients that could not otherwise have been undertaken under the STEP database project,” says the group. The scheme will allow end-users to include the excipients of their choice into the database on a priority basis.
“Sponsor an excipient means additional resources to fund services that can help us in our daily tasks of collecting and curating information,” according to EuPFI. Sponsors will support the scientific community to facilitate the development of safe drugs for children and will also be listed with their logo on the EuPFI STEP database page.
Anyone interested in getting an excipient sponsored should contact admin@eupfi.org with a proposed excipient and deadline, and EuPFI estimates the sponsorship costs will be on a sliding scale from around £1,000 to £5,000 – dependent on the number of references per excipient.
Could desktop assessment reduce EU on-site inspections?
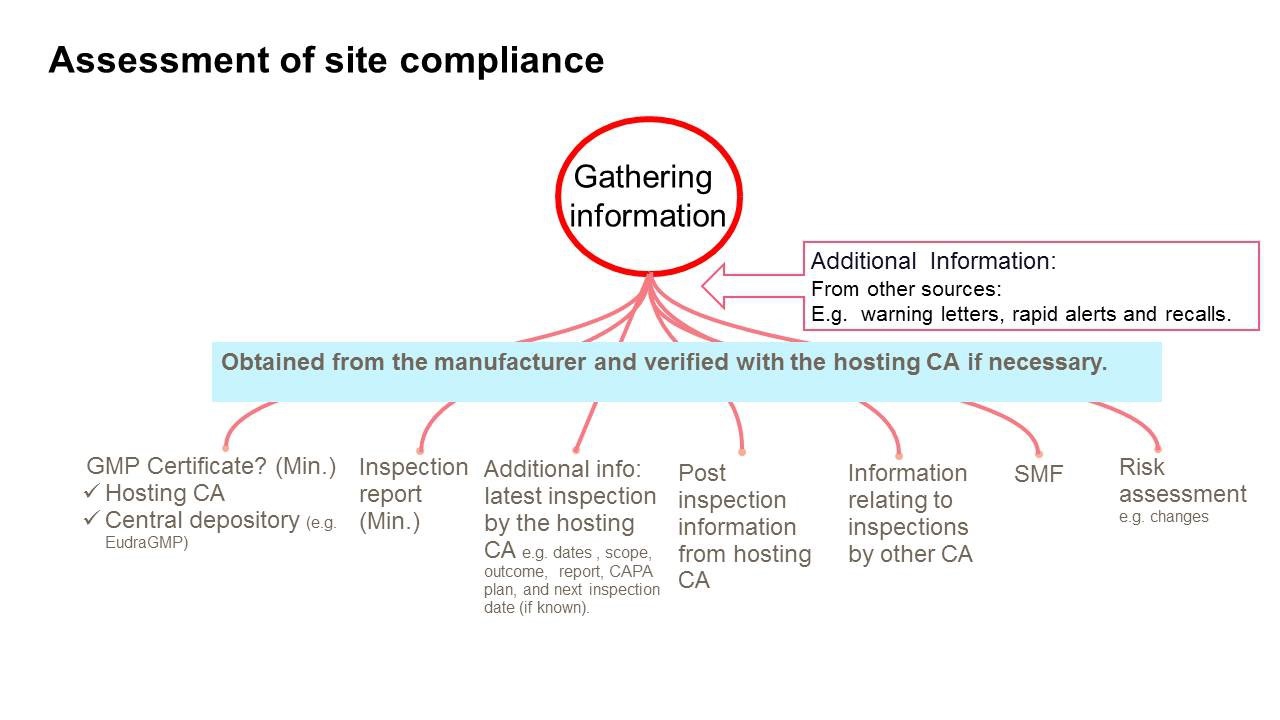
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) has adopted new, non-binding guidance on Good Manufacturing Practice (GMP) inspections which includes a new process for desktop assessment of GMP compliance.
The desktop assessments would help regulators prioritise resources for GMP inspections, where demand “far exceeds what any one [competent authority] can accomplish.” The new form of assessment would be deployed “instances where an acceptable level of GMP compliance can be confirmed and assured from the activities of another CA or CAs without the need for an on-site inspection,” says PIC/S in its latest newsletter.
A schematic of the desktop assessment appears in the picture on top of the article.
PIC/S says the adoption of the new guidance highlights efforts to improve sharing of information between authorities.
PIC/s formally adopts EC's excipient GMP risk-assessment guidelines

At its meeting in April, the Pharmaceutical Inspection Co-operation Scheme (PIC/S) officially adopted the transposition of the European Commission Guidelines on the formalised risk assessment for ascertaining the appropriate good manufacturing practice for excipients of medicinal products for human use.
The guidelines will enter into force on 1 July 2018, on a voluntary basis for non-EU/EEA PIC/S participating authorities. According to PIC/S, “transposition of these guidelines further strengthens harmonisation between PIC/S and the EU and are in essence equivalent with some minor editorial differences. The guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities is also closely linked to revised Chapter 5 of the PIC/S GMP Guide.”
As a reminder, IPEC Europe has also worked hard to develop guidance to help organisations meet the regulatory requirements for GMP for excipients, for example with an update to the Joint IPEC-PQG Good Manufacturing Practices Guideline last year and the publication of our ‘How-To' document on Risk Assessment in 2016.
PDG holds first videoconference meeting

The Pharmacopoeial Discussion Group (PDG) has continued to change its procedures, and in April held its first videoconference to bring together representatives of the European Pharmacopoeia (Ph.Eur.), Japanese Pharmacopoeia (JP), the United States Pharmacopoeia (USP) and the WHO (International Pharmacopoeia) as an observer.
It follows a PDG decision to review its meeting formats to facilitate “exchanges between the experts in the regions in order to resolve issues on specific PDG topics.” Face-to-face meetings will still take place once a year, with one interim video conference. These meetings will focus on strategic direction setting.
The PDG also agreed to launch a pilot phase to try a prioritisation scheme for excipient monographs and general chapters. This trial phase concerns 10 excipient monographs and five general chapters; at the next PDG face-to-face meeting it will be decided whether or not to expand the prioritisation scheme to the other items on PDG work programme.
The next annual face-to-face PDG meeting will be hosted by Ph. Eur. on October 2-3, 2018, at the EDQM premises in Strasbourg, France, and of course the IPEC Federation will be there to observe and contribute to the proceedings.
What do you think should be on the agenda at PDG? Don't hesitate to let the IPEC Europe Secretariat know here.
EMA starts new consultation on ICH Q3D
The European Medicines Agency (EMA) has opened a new consultation on ICH Q3D Elemental Impurities – which extends to 16 August. The intention is to carry out some minor housekeeping to the guidance, including error correction of the permitted daily exposure (PDE) for Cadmium by the inhalation route from 2µg/day to3 µg/day, and does not include any significant changes.
For reference, the new consultation document appears here.
New EDQM guideline "How to read a CEP"

The European Directorate for the Quality of Medicines & Healthcare (EDQM) has drawn up a new guideline entitled “How to read a CEP” or Certificate of Suitability.
The aim is "clarifying the information to be concluded from a Certificate of suitability to the Monographs of the European Pharmacopoeia (CEP) for industry and the competent authorities," it says. Marketing Authorisation (MA) applicants are advised to read existing guidance published by the competent authorities in their countries, or to contact them directly for advice, when using a CEP to replace the respective quality part of the CTD dossier related to that given source, or in any variation.
Recommended reading

EMA annual report published
The European Medicines Agency (EMA) has published an overview of the work of the European Medicines Agency (EMA) and highlights last year’s major achievements in protecting and promoting public and animal health in the EU. Some of the agency’s main projects and initiatives are highlighted, including the first public hearing, the launch of the new EudraVigilance system, the first anniversary of PRIME (PRIority MEdicines) and the new framework and action plan for academia – as well as the usual data on inspections and compliance.
European Medicines Agency
UK pharma cheers EU science announcement
Amid all the chaos and confusion of Brexit, the UK pharma industry finally has some good news to latch onto – it needn’t be excluded from EU’s flagship Horizon science programme. The European Commission has just published its budget proposal for the next phase of Horizon, setting aside almost €98bn for Horizon Europe - which gets underway in 2021 after the current Horizon 2020 comes to an end – and will extend out to 2027.
PMlive.com
An important Brexit silver lining: the UK commits to align with EU rules on clinical trials
We welcome news that the UK will seek to align as closely as possible with the EU rules on clinical trials following Brexit. It means the EU and UK should be able to continue to collaborate and work together effectively after Brexit, draw on each other’s expertise and continue to run world class multi-member state trials, which will benefit patients in the UK, the EU and internationally. This also ensures that patients will gain access to trial medicines without delay.
LeighDay.co.uk
EU-US mutual recognition: FDA gives two more EU countries seal of approval
Following a positive assessment by the US Food and Drug Administration (FDA), Lithuania and Ireland have today joined the list of EU countries recognised as capable to carry out good manufacturing practice (GMP) with regard to inspections of sites that manufacture human medicines. The FDA already recognised eight EU countries on 1 November, 2017 and four additional countries on 1 March, 2018. This brings the total number of countries accepted by the FDA to 14. The remaining member states will be assessed by the FDA on a rolling basis, to be completed by 15 July 2019.
European Commission
Brexit: EMA still clear on timelines
Despite the currently discussed "transition period", the European Medicines Agency EMA has emphasised that industry should be well prepared even for a so-called "hard Brexit". This was one of the main statements made in an industry stakeholder meeting on Brexit and operation of the centralised procedure for human medicinal products, held on 23 March 2018.
ECA GMP news
Events Calendar

Here is a round-up of forthcoming events of interest to suppliers and users of excipients. Please let the IPEC Europe Secretariat know if we've missed one.
ExcipientFest Asia
Beijing, China - 19-20 July, 2018
More information here.
10th EuPFI Conference
London, UK - 11-13 September, 2018
More information here.
APV/IPEC Europe Excipient Conference 2018
Cologne, Germany - 18-19 September, 2018
More information here.
CPhI Worldwide
Madrid, Spain - 9-11 October, 2018
More information here.
|