Editorial

Dear Members,
As we approach the second half of the year and publish this June-July issue, we find ourselves heading for a well-deserved summer break. No doubt this has been and may continue to be, a very demanding period for you. However, I hope you are able to get some respite from meetings, and mid-year reviews, made even more exacting because of the demands imposed by COVID-19.
Our Secretariat is fully engaged and preparing for a rentrée full of activities. Meanwhile, there are several features and news in this edition which I’d like to highlight.
A new infographic developed with APIC and EFCG, showcases the importance of the pharmaceutical supply chain in Europe and the need for a coherent pharmaceutical strategy. This was prepared with a view to initiate discussions with DG Sante as part of our initial response to the European Commission’s ‘Pharmaceutical Strategy -Timely patient access to affordable medicines’. This is a great opportunity to further our cooperation and engagement with industry stakeholders, not least to get the novel excipients on the broader agenda as a significant contributor to innovation in medicines. And now here’s the first of my appeals for this edition! As ever, we need volunteers to help author our complete position on this significant initiative so if this interests you, please get in touch with the Secretariat so we can get you involved.
Similarly, I’d like to draw your attention to the survey “Novel Excipients – Why don’t we use them” which we’ve just reissued. As we try to build the case for a dedicated regulatory pathway for novel excipients in Europe, we must gather information with as many examples as possible from the user community, i.e. drug formulators and pharmaceutical companies. This is a very important topic to IPEC Europe so please share the survey within your network so we reach as many as possible who can provide these data. We look forward to hearing from you!
And finally, still in the regulatory arena, the subject of nitrosamines has occupied much time in the past 12 months. I hope that you’ll share the proposal to create a data base of information on excipients widely which should greatly facilitate the burden of ‘user’ risk assessments, the need for which is spreading globally.
Meanwhile, please enjoy the many other features in this edition and see you in September!
Frithjof Holtz
IPEC Europe Chair
EDQM and IPEC Europe: virtual meeting

IPEC Europe met virtually with EDQM on 17 June to exchange views on current activities and topics of interest.
The agenda covered the co-processed excipients, the nitrosamines, also elemental impurities and the IPEC project on multicompendial convergence. The Directorate also reported that next steps on the draft revised General Chapter on Substances for Pharmaceutical Use (2034), will be communicated in autumn.
EDQM confirmed that excipients are not in the scope of the proposed Ph.Eur. General Chapter 2.4.35 on “Extractable elements in plastic materials for pharmaceutical use”.
If any member would like more information on these topics, please contact the Secretariat: info@ipec-europe.org
Nitrosamines: database of information on excipients
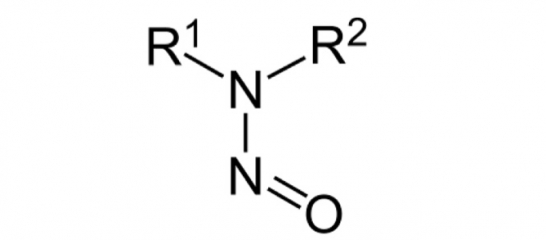
As many will appreciate, there are significant concerns over the potential presence of N-Nitrosamines in pharmaceuticals. Starting with issues of contamination of Valsartan with an N-Nitrosamine impurity , N-Nitroso dimethylamine (NDMA), this has become a concern for all synthetically manufactured APIs through the publication by the EMA of the Article 5(3) process.
Although initially focused on risk pertaining to the synthesis, concerns have widened to include other factors including formation during formulation and packaging. Focusing on the drug product manufacturing-related risk, this centres on the presence of a secondary amine (typically the drug itself or a significant degradant) and the theoretical reaction with nitrites present in excipients. Investigations continue into the viability of the reaction itself, but an obvious factor is the level of nitrites in excipients.
To address this and hopefully put this ‘risk’ into proper context, an initiative has been set up to establish a database of knowledge of nitrite and nitrate levels in common excipients. Gaining a clear understanding of the actual levels of nitrite is vitally important to avoid routine testing of nitrites and N-Nitrosamines based simply on a postulated risk.
This is where collaboration between the pharmaceutical industry and excipient suppliers can prove highly effective.
We need you!
We are seeking help from excipient manufacturers to share whatever data they have in relation to nitrite and nitrate levels in the materials they supply. All data will be anonymised; this is solely about gaining a greater understanding about this situation through gathering data and nothing else. If you’d like to participate, please contact the Secretariat: info@ipec-europe.org
Updates from IPEC Europe: Board and Committees

Board
The second IPEC Europe Board meeting of the year was held by webconference on 24 June. Here are some highlights:
- Progress on the 2020 IPEC Europe objectives as agreed at the AGM in February was discussed in the context of the current pandemic. Some adjustments in target dates were made.
- There was good news! Two new members joined (Selectchemie and Zerion) IPEC Europe.
- The Board was also debriefed on the EDQM teleconference, see above for details.
- Preparation for the IPEC Federation mid-year reviews were made which this year was held remotely due to travel restriction. IE was represented by F Milek (IPEC Federation Chair), F Holtz and K Hughes.
- The events calendar has been severely disrupted by Covid19. The APV Conference has been postponed to 2021 at the same venue in Frankfurt (DE). Webinars will be organised in collaboration with APV as an alternative. The Board will decide in September the best way forward for the 2021 IE Annual Forum.
Quality and Regulatory Affairs Committee (QRAC)
The Core team reunited virtually on 2 July before the summer break. The Committee reviewed the 2020 Objectives and the actions list.
The Committee had the opportunity to discuss the new DG SANTE initiative on ‘Pharmaceutical Strategy -Timely patient access to affordable medicines’. Members debated the impact of the Chinese NMPA technical guidelines on the evaluation of nitrosamines in drug products and the EMA “Lessons learnt” document on nitrosamine impurities in sartans.
Additionally, the team considered the opportunity to create a nanomaterials task force to create a summary document of existing positions from regulators.
Some time was devoted to discussing on the EXCiPACT remote auditing initiative, the current status of the titanium dioxide debate and the latest IPEC Federation position paper on GMP documents for pharmaceutical excipients.
Titanium Dioxide, microplastics and nanos are recurring items on the QRAC agenda. The current regulatory issues were discussed including the possibility of developing an IPEC Europe position on nanomaterials and next steps related to the SEAC draft opinion opened for comments.
Please contact the Secretariat if you have questions: info@ipec-europe.org.
IPEC Europe Task Forces, activities aplenty!

Multicompendial Convergence (MCC)
The team selected two examples (Titanium Dioxide and Propylene Glycol) to test the multicompendial convergence concept. By comparing analytical methods across ChP, JP, Ph Eur, and USP, could one method be used to confirm compliance with all pharmacopoeias? Initial results are encouraging but more work needs to be done before developing the desired industry best practice on this topic.
Lactose Interest Group (LIG)
One objective of LIG is to design the ideal monograph for Lactose which could be helpful in driving global harmonisation in future. This is work in progress.
The team drafted a position paper based on the premise that although lactose is derived from an animal derived material (whey), lactose as a pharmaceutical grade is a low-risk excipient in oral dosage forms.
Co-Processed Excipients (CPE)
Over the recent years, the GMP status of CPEs has been the subject of much debate. These materials play an increasingly important role in the innovative use of excipients in pharmaceutical formulations. Hence, IPEC Europe is eager to do what it can to make it clear that as excipients, they should be manufactured under GMP determined through a formalised risk assessment (EC Guidelines 19 March 2015; 2015/C 95/02).
Plans are under discussion to develop the appropriate arguments to engage with regulators and other industry associations.
Webinar: IPEC Federation Data Integrity position paper

IPEC Europe held its second webinar of the year on 9 July, covering the principles of Data Integrity and describing the IPEC Federation Position paper on how these principles apply to excipients.
The online event, presented by Dale Carter, Head of Quality Silica BS in North America at Evonik Industries, attracted more than 60 attendees. Dale gave an overview on ALCOA+ concepts, and where these principles can be found in the IPEC Excipient Good Manufacturing Practices guide. This insightful presentation triggered an interesting Q&A session.
IPEC Europe Members can access the recording of the webinar free of charge here.
IPEC Federation – New position paper available on GMP Documents for excipients

IPEC Federation published a new position paper titled “Pharmaceutical Excipients GMP Documents supported by the IPEC Federation”.
This paper explains the purpose of the different good manufacturing practice (GMP) guides and standards available for excipients, and how they may be used. Excipient manufacturers can better understand the documents and decide which is the most appropriate to adopt for their purpose.
The Joint IPEC PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients, EXCiPACT™ GMP Annex and NSF/IPEC/ANSI 363 excipient GMP standard are described, along with questions to help the excipient manufacturer select the document to align with. IPEC points out that an excipient manufacturer should implement a Quality Management System designed around one of the three and should monitor future developments, so it is transparent to both customers and auditors which requirements are being followed.
The position paper is available for download via the websites: www.ipec-federation.org and www.ipec-europe.org. For further information contact the IPEC Federation Secretariat at +32 2 213 74 40 or write to info@ipec-federation.org.
Announcing two new IPEC Europe members: Zerion ApS and SelectChemie AG

IPEC Europe is delighted to welcome Danish drug delivery specialist Zerion ApS and Swiss excipient supplier Selectchemie AG as new members.
Zerion is a pharmaceutical company that has developed a proprietary formulation platform called Dispersome, that improves the solubility and bioavailability of oral drug products. The platform is based around a class of novel excipients to formulate poorly soluble small molecule drugs into stable drug delivery system with a high drug load. Zerion aims to simplify the development of new treatment options for patients across all small molecule therapy areas.
You can learn more about the company on their website: www.zerion.eu.
Selectchemie is an independent Swiss company serving the pharmaceutical and human nutrition industries since 1969 as a premier supplier of high-quality ingredients. The company offers services to ensure the complete long-term provision of raw materials for the pharmaceutical and food industries.
For further information you can visit www.selectchemie.com.
The full list of IPEC Europe members is available on the IPEC Europe website.
Infographic: the Pharmaceutical Supply Chain in Europe
IPEC Europe joined with EFCG (European Fine Chemicals Group) and APIC (Active Pharmaceutical Ingredients Committee), sector groups of CEFIC, to publish an infographic to describe the pharmaceutical value chain in Europe and what is needed to make it resilient and sustainable.
Click here for the full version of the new infographic on “The Pharmaceutical Supply Chain in Europe”!
IPEC Europe Excipient Conference 2020 organised as webinar series

Your health and safety come first! Due to the uncertainty caused by Covid-19, IPEC Europe and APV took the difficult decision to postpone the 2020 Conference to September 2021 still in Frankfurt, Germany.
As an alternative, we will organise a webinar series on 17-18 September 2020 to provide you with the latest insights on pharmaceutical excipients and IPEC Guides.
• Technical Unavoidable Particles – A practical introduction to the IPEC guideline - Darek Lewin, J. Rettenmaier & Söhne (JRS Pharma) GmbH + Co KG, Germany
• The Importance of Excipients in Continuous Manufacturing - David Schoneker, Black Diamond Regulatory Consulting, LLC, U.S.
Please mark your calendar! Registrations will open in the second part of August.
Stay tuned and see you in September!
Survey: Novel excipients – why don’t we use them?

Do we need them?
- Novel excipients foster innovation and better treatment options.
- Innovator and generic companies need novel excipients to increase the bioavailability of poorly soluble APIs or as a base for new drug delivery systems.
- Novel excipients help to re-formulate drugs to improve quality and safety.
Why aren't they used?
- A USP survey reported that regulatory issues were the most frequently cited challenges not to use novel excipients.
- In Europe commercially sensitive and proprietary information is not protected building a barrier to innovation.
What needs to happen?
- Bring Europe in line with other global markets, establish a regulatory pathway for novel excipients (e. g. Master File system).
- Innovator and generic companies must declare their regulatory needs for novel excipients to drive innovation.
How can I help?
- Complete this survey by 31 July 2020.
- Provide examples where innovation was blocked because a novel excipient could not be selected due to regulatory barriers.
Complete the Survey
Please note: this survey is intended for drug formulators and excipient users.
Regulatory News: Europe

European Union
European Commission consults on roadmap to pharmaceutical reforms
The European Commission is looking to revamp parts of the regulatory framework for medicines in the EU to address shortages, access to medicines and innovation, according to a roadmap document released for consultation.
European Commission / GMP Publishing
European Medicines Agency (EMA)
EMA explains GMP and GDP flexibilities amid COVID-19
EMA, the European Commission and the Heads of Medicine Agencies updated their guidance for pharmaceutical companies on adaptations to the regulatory framework to clarify the exceptional change-management process in place to reduce the risk of shortages or disruption of supply of crucial medicines for COVID-19. The update also introduces further temporary flexibility on good-manufacturing- and good-distribution-practice inspections and new guidance on inspections of plasma collection centres.
European Medicines Agency / RAPS
EMA Joint Strategy for the upcoming 5 years
EMA and the Heads of Medicines Agencies (HMA) have developed a joint strategy for the next five years that is released for a two-month public consultation. The draft strategy details how the European medicines agencies’ network can continue to enable the supply of safe and effective medicines that meet patients’ needs in the face of challenges posed by ever-accelerating developments in science, medicine, digital technologies, globalisation as well as emerging health threats.
European Medicines Agency
Emer Cooke becomes new EMA Executive Director
The Management Board of the European Medicines Agency nominated Emer Cooke as the new Executive Director from a shortlist of candidates provided by the European Commission. Ms Cooke, a former director of the Regulation and Prequalification Department at the WHO, has thirty years’ experience in international regulatory affairs. In her immediately preceding role, she leaded WHO’s work on health technology regulation, standards and regulatory systems strengthening.
European Medicines Agency
EMA Annual Report 2019 published
EMA released its Annual Report 2019 which offers insights on plans for an EU real-world data network to support innovation and public heatlh. The report also includes some of the Agency’s main initiatives and challenges, including the successful relocation to Amsterdam, the development and finalisation of the ‘regulatory science strategy to 2025’ and the response to the discovery of nitrosamine impurities in medicines.
The 2019 report is also available in a digital version at the address https://www.ema.europa.eu/en/annual-report-2019.
PDF version
European Directorate for Quality of Medicines (EDQM)
Pharmeuropa 32.3 released: comments until 30 September
The latest issue of Pharmeuropa contains 48 drafts published for review and comments. It includes also the recently proposed new Chapter 2.4.35 “Extractable elements in plastic materials for pharmaceutical use” already published in Pharmeuropa 32.2 to give stakeholders more time to comment. The full list is available here.
European Chemicals Agency (ECHA)
ECHA’s Committee for Risk Assessment (RAC) supports restricting intentional uses of microplastics
The Committee for Risk Assessment adopted its opinion on the Agency’s proposal to restrict the intentional use of microplastics added to products.
Biodegradable polymers: ECHA’s proposal set out specific test methods and pass criteria for identifying biodegradable polymers, which are excluded from the restriction. RAC wanted to see greater evidence that microplastics are biodegradable in the environment (e.g. soils, marine environment, freshwater).
Use of microplastics as infill material on artificial turf pitches: RAC recommended a complete ban after a transition period of six years as there was incomplete information on the effectiveness of risk management measures. A ban would also be more effective than risk management measures in preventing environmental releases in the long term.
The definition of ‘a microplastic’: ECHA proposed a lower size limit of 100 nanometres for a microplastic as analytical methods for detecting microplastics in products (i.e. mixtures) are still in development. RAC recommended that a lower size limit is not necessary as the potential restriction can also be enforced in other ways, such as by looking at raw materials in supply chains.
ECHA
Candidate List update: Four new hazardous chemicals to be phased out
The addition of three plastic additives and a cosmetics preservative used also in pharmaceutical products, butylparaben, has brought the EU’s list of substances of very high concern to 209.
ECHA
Regulatory News – Rest of World

U.S. Food and Drug Administration (FDA)
FDA Issues Guidance on GMP Considerations for Responding to COVID-19 Infection in Employees in Drug and Biological Products Manufacturing
This FDA guidance provides recommendation to pharmaceutical manufacturers concerning GMP requirements and expectations to prevent contamination of drugs and exclude sick workers from engaging in drug manufacturing.
FDA / IPEC-Americas
FY 2020 Generic Drug Regulatory Science Initiatives Public Workshop: Presentations available
The FDA held a workshop in May to provide an overview of the status of the current FY 2020 Generic Drug User Fee Amendments (GDUFA) Science and Research Priorities. Presentations and recording of the sessions are available
FDA / IPEC-Americas
CDERLearn Training and Education: free resources
FDA offers online material as part of their CDERLearn Training and Education Program, with a special section containing courses for the pharmaceutical industry.
FDA
U.S. Pharmacopoeia (USP)
USP Novel Excipients Survey: Stakeholders’ Views on the Current State of Excipient Innovation
Survey results indicate that the current regulatory approval pathway for excipients creates a challenge for the use of novel excipients, according to USP.
PharmTech
2020 Convention Governance meeting outcomes
The outcomes of the first ever virtual USP Convention Meeting, celebrating the 200th Anniversary of the US Pharmacopoeia and the new strategy for the five-year period up to 2025, are now available to the public.
USP
International Council for Harmonisation (ICH)
ICH Global harmonisation efforts continued through virtual meetings
The ICH met virtually on 27 May, in place of the face-to-face meetings that were to take place in Vancouver, Canada. Highlights of the meeting include the progress tracking of existing ICH guidelines, agreement on new ICH harmonisation activities and updates on MedDRA.
The next ICH Assembly meeting is planned to take place on 17-18 November 2020 in Athens, Greece.
ICH
Chinese Pharmacopoeia (ChP)
2020 Chinese Pharmacopoeia into force end-of-year
IPEC China informed that the Chinese Pharmacopoeia Commission has published the ChPa 2020 and will enter into force as of 30 December 2020. According to our Chinese colleagues, the English version may be issued up to two years later. An online version of the ChP is not available now but is under consideration by the Chinese Pharmacopoeia Commission.
International Coalition of Medicines Regulatory Authorities
COVID-19 – re-cap page
During the ongoing COVID-19 pandemic, the International Coalition of Medicines Regulatory Authorities (ICMRA) is acting as a forum to support strategic coordination and international cooperation among global medicine regulatory authorities. After the collective statement published in April, ICMRA members committed to prioritise COVID-19 clinical trials to expedite vaccines development and approval.
ICMRA
Calendar

All meetings are scheduled to take place online (TC) until further notice.
|
Group
|
2020
|
|
IPEC Europe Board
|
23 September
25 November
|
|
Good Distribution Practices Committee
|
29-30 September
1-2 December
|
|
Quality & Regulatory Affairs Committee
|
27 October
3 December, TC
|
|
Multicompendial Compliance TF
|
1 September, F2F/TC
|
|
Lactose Interest Group TF
|
22 September, TC
|
|
Co-processed excipients TF
|
TBC
|
Recommended readings

Excipient industry pursues quality improvement efforts to reduce dependency on imports
An interview with Vishakha Metkar, Chairperson of IPEC India, on the status quo of the Indian pharmaceutical excipient industry, its challenges, and opportunities. The role of the IPEC Federation and its guides is beneficial to manufacturers and suppliers in the country.
PharmaBiz.com
UK expected to break away from ECHA and EU REACH post-Brexit
The UK will not seek associate membership of the ECHA after it departs the EU customs union next year, according to a UK Member of Parliament.
ICIS / Chemical Watch
Ensuring a healthy supply chain throughout the COVID-19 crisis
Covid-19 has presented an unprecedented challenge, and not least to the supply chains that fuel so many aspects of our lives. Ensuring uninterrupted access to quality medicines, around the world, has been the immediate focus of our industry as we navigate this strange time, says Caroline O’Brien, Chair of PSCI (Pharmaceutical Supply Chain Initiative).
Global Pharma Insights
Indian Department of Pharmaceuticals proposes to oversee drug regulator
The Department of Pharmaceuticals has proposed that the oversight of the Central Drugs Standard Control Organisation be transferred to it, as the Indian government plans a major overhaul of the country’s drug authority. According to media reports, concerns had been raised at the highest levels of the government over the ability of the regulatory framework to keep up with the demands of industry and scientists, triggering the move to revamp the drug authority.
India Times
Inactive ingredients, the unsung players in medicines
The Therapeutic Goods Administration (Australian Government, Department of Health) publishes an article to inform citizens of the role of excipients in medicines at a time when the authority announces plans to publish additional information about medicine formulation on its website. ARTG Summaries for all medicines list both the active ingredients and non-active (excipient) ingredients.
Australian Government
A Welcome Change: The Benefits of Reformulation
Reformulation strategies can provide drug developers with a head start to achieve promising options that benefit the patient.
PharmTech
Complying Confidently? Learning Lessons from Nitrosamine Impurities
Industry must never become complacent about the safety of drug products and should seek to continually perform surveillance even if the drug is well-established.
PharmTech
Events

Here is a round-up of forthcoming events of interest to suppliers and users of excipients. Please let the IPEC Europe Secretariat know if we've missed one.
12th conference of the European Paediatric Formulation Initiative (EUPFI)
Virtual conference – 9-10 September 2020
More information here
2020 ISPE Europe Annual Conference
Virtual conference – 16-17 September 2020
More information here
IPEC Europe Excipient Conference Webinar series
Webinar series – 17-18 September 2020
More information here
POWTECH 2020 Special Edition
Nürnberg, Germany – 30 September – 1 October 2020
More information here
CPhI: Festival of Pharma
Virtual Conference – 5-16 October 2020
More information here
23rd APIC/CEFIC Global GMP & Regulatory API Conference
Amsterdam, The Netherlands – 28-30 October 2020
More information here
4th PQRI Workshop on ICH Q3D Elemental Impurities Requirements
Virtual Conference – 9-10 October 2020
More information here
|