Editorial

Dear Members,
As I dwell on the introduction for this edition of Excipients Insight, the current pandemic in which we find ourselves is foremost in our minds. It is having wide-reaching impact on so many aspects of our lives and let us hope better days are not too far away.
I know that many of you as key workers will be involved in activities to ensure the uninterrupted supply of excipients and medicines. These efforts are pivotal to prevent shortages at a time when availability issues can have an even greater impact. It is an extremely demanding time but how the management of this pandemic has challenged norms could present positive outcomes in the form of learning. Let’s hope that in the aftermath of this very traumatic and tragic experience these changes to our ways of working could be permanent changes for good.
Meanwhile, business carries on as best it can but clearly dominated by COVID-19. Our staff in Brussels are operating from home and successfully managed the launch of two new guides and the long-awaited position paper on Data Integrity, which you can read about in this issue. Also, our programme of events is being heavily impacted with some being rescheduled (Making Pharmaceuticals UK) and others still pending further announcements on social distancing. On-going regulatory matter are still very active, with developments on E171 Titanium Dioxide and Nitrosamines requiring attention. We have joined pan-industry discussions with EDQM on what IPEC Europe and others believe need to be prioritised and considered in these special circumstances. Further reports will be provided but for more details now, please get in touch.
Hopefully, by the time we next publish Excipients Insight, some degree of normality will have been re-established, but until then, keep well and safe.
Frithjof Holtz
IPEC Europe Chair
IPEC Federation: position paper on Data Integrity and new bulletin

Position Paper: Data Integrity for Pharmaceutical Grade Excipients
This Position Paper was developed to supplement published regulatory guidance defining data integrity requirements for drug substances and medicinal products, some of which are not easily adaptable to the manufacture of excipients.
Data integrity is fundamental to the application of GMPs and clearly relevant to excipient manufacturing; however, data integrity controls for excipients may be different from controls used for the manufacture of drug substances and medicinal products.
The IPEC Federation believes that data integrity controls for excipient manufacturing and distribution processes should be commensurate with risks.
Read this Position Paper on the IPEC Europe and IPEC Federation websites.
IPEC Federation Connect: activites and news from the Federation
The first edition of the bulletin of the IPEC Federation, “IPEC Federation Connect”, was published in early March.
The biannual bulletin aims to provide all IPEC sister associations with a periodic round-up of activities and events, and enhance the awareness of the Federation with IPEC members and the broader pharmaceutical-realted industries.
The March 2020 issue can be downloaded on the IPEC Federation website.
Updates from IPEC Europe: Board and Committees

Board meeting
Due to the pandemic situation, the Board held its first meeting of the year remotely on 19 March.
As per the Article 16 of the IPEC Europe Articles of Association, the following officers were confirmed in their respective role:
- Frithjof Holtz (Merck), IPEC Europe Chair
- Frank Milek (Aug. Hedinger), IPEC Europe Vice-Chair
- Karsten Diehl (BASF), IPEC Europe Treasurer
- Karine Roth (Novartis), IPEC Europe Secretary
The Board reviewed the 2020 objectives adopted by the AGM on 7 February and defined milestones and timelines.
Sponsors were assigned for each objective:
- Communications: Kevin Hughes (Colorcon)
- Global collaborations: Frithjof Holtz (Merck) and Frank Milek (Aug Hedinger)
- Regulations: Karsten Diehl (BASF)
- Knowledge sharing: Amina Faham (DuPont)
- Compendial Compliance Frithjof Holtz (Merck)
- Membership and Recruitment: Mahmud Yunis (BIOGRUND)
The IE infrastructure is a recurrent activity that is under the supervision of K Roth, Board Secretary
Johanna Eisele, Quality & Reg Aff Committee (QRAC) Chair, joined the meeting in part to discuss the EMF for novel excipients initiative, the titanium dioxide situation and the structure of the QRAC.
The Board also discussed the 2020 events and considered restructuring the annual forum and the AGM to facilitate the participation of the members. The current and futures IPEC Federation activities were also reviewed.
Call with EDQM on the impact of COVID-19 pandemic
On April 3, EDQM invited several European industry associations, including IPEC Europe, to exchange views and concerns over the COVID-19 pandemic and its impact. Upon IPEC Europe’s request, the Directorate informed that there are no plans to extend commenting periods. Nonetheless, companies and associations may request an extension for specific products, respectively via their national pharmacopoeias or EDQM. The Directorate works to ensure the continued availability of the necessary Reference Standards. EDQM organised a follow up call on 5 May where the above was confirmed. The next call is scheduled on 10 June.
Notes of these calls are available upon request to the Secretariat.
Good Distribution Practices Committee
The Good Distribution Practices (GDP) committee organised its latest virtual meeting on 29 April.
The revision of the 2011 IPEC GDP Audit Guide is progressing, the committee revised and commented several chapters received from IPEC-Americas. Discussions are ongoing to agree upon a final draft before the summer break.
Progress was also made with another key project, the transportation of excipients. Members discussed on what data is required to determine risks related to the transport of excipients. Several committee members agreed to share information on actual transport conditions during monitored shipping. The committee plans to consolidate data as a basis for defining requirements for collecting additional data in future meetings.
Furthermore, GDP Committee members discussed and commented the draft project charter of the IPEC GDP Guide “How to amendment” received by IPEC-Americas. This project is planned to start after the finalisation of the GDP Audit Guide revision.
The next meeting will take place virtually on Monday 8 June. For further information, please contact the Secretariat.
Quality and Regulatory Affairs Committee (QRAC)
Twenty-eight QRAC (Core Team) members joined the second meeting of the year on 23 April.
The 2020 Objectives defined in Aix-en-Provence were reviewed and discussed. The Committee continues its support to the creation and revison of IPEC guides, and agreed to create ad hoc, topic-driven task forces focussing on specific topics. Currently, three task forces on microplastics, nitrosamines and nanoparticles are operational, with an additional task force being formed to support an opinion on the applicable GMP for drug products relating to co-processed excipients.
Some time was devoted to evaluate topics and potential speakers for webinars, and updates on novel excipients activities from other stakeholders.
Please contact the Secretariat if you have any questions on any matter.
The next QRAC Core Team meeting is planned on Thursday 2 July.
IPEC Guides: Excipient Information Package and Composition Guide now available to members

In April, the IPEC Federation announced the availability of two IPEC Federation revised guides, the Excipient Composition Guide and the Excipient Information Package (EIP) Guide.
The two guides are currently available to IPEC members, and will be released to the general public on 22-23 July. Being a member of IPEC Europe entitles you to exclusive access to these guides and many other advantages.Members of IPEC Europe, you can obtain the guides from our Member’s Lab, under Members / Guidelines and Useful documents.
If you do not have your log-on credentials yet, please contact the Secretariat.
The IPEC Excipient Composition Guide
The Composition Guide provides an approach for excipient manufacturers to establish a composition profile for a pharmaceutical excipient, intending to provide strategies for assessment of the overall composition of their excipient.
Originally published in 2009 by IPEC-Americas and IPEC Europe, the guide has been revised to update the references conteained therein and represent inputs from all IPEC members in the Americas, China, Europe, India and Japan, thus becoming an IPEC Federation guide.
Access the IPEC Excipient Composition Guide
The IPEC Excipient Information Package (EIP) Guide
The primary goal of the EIP guide is to facilitate sharing of excipient supplier’s information with users in a standardised way rather than completing individual questionnaires and surveys.
The EIP consists of three different documents: Product Regulatory Datasheet, Site Quality Overview, and Supply Chain and Security Overview. Templates for each of these documents are available separately.
The EIP Guide was originally published in 2005, and subsequently revised in 2009 and 2013.
Access the IPEC Excipient Information Package (EIP) Guide
First call for the 2020 IPEC Europe Excipients Conference

IPEC Europe and APV are delighted to invite you to our 9th annual conference on pharmaceutical excipients.
The conference will focus on regulatory guidelines relating to pharmaceutical excipients and technology.
Excipients play a central role in the drug development process, in the formulation of stable dosage forms and in their administration.
Excipients can contribute to the processing of drug delivery systems.
Since excipients are key parts of the formulation of medicines, we will open the second day with an outlook into the role of excipients to the year 2025 and beyond. Afterwards we will take the opportunity to listen to the new developments on excipients at EDQM and USP. Besides this we will highlight the role of excipients in the development of topical and intraoral drug delivery systems.
Continious manufacturing presents a flexible approach to oral solid dosage form production and meets the industry’s demands for faster product development, reduced costs and increased manufacturing flexibility. We will learn the suitability and specific requirements of excipients for this new manufacturing process. Compendial specifications may not address all the physiochemical properties that can have an affect on manufacturability and final product quality.
The versatility and flexibility of 3D printing technique offers a large spectrum of advantages and will likely implement substantially improved medications in near future.
Here, we will listen to a presentation of modified release tramadol printlets (3D printed tablets) with alcohol-resistant and abuse-deterrent properties prepared by direct powder extrusion three-dimensional (3D) printing.
The identification of contaminants on the surface of excipients used in the pharmaceutical industry with non-destructive techniques is a challenging procedure. Here we will present a modern technique based on Ultrasound Acoustic Atomic Force Microscope (UAAFM).
We are looking forward to welcoming you in Frankfurt.
OBJECTIVES
The primary goal of the conference is to highlight current hot topics in the field of pharmaceutical excipients. Take opportunity to share your experiences and discuss with colleagues of pharmaceutical industry, academics, authorities as well as with manufacturer and distributors of pharmaceutical excipients. This year again, we offer you to arrange your individual conference focus by choosing 2 of our three practical sessions on the first day:
- Data Integrity
- Internal analytical method harmonisation
- Managing appropriate transport conditions for excipients – current experiences
Futher hot topics of the conference in this year will be:
- Excipient Regulation in Europe and Emerging Countries
- Change Control - What is different between pharma and excipient industry
- Excipient specific information and data required for drug product registration and supplier qualification
- The role of excipients in topical drug delivery
- 3-D printing
- Intraoral drug delivery
- Continuous manufacturing
- Data Integrity - current status of regulation
- Identification of contaminants in excipients using a non destructive technique
REGISTER NOW
EXCIPACT announces a procedure for audit postponement and remote audits

In response to the COVID-19 pandemic and following their position paper of 24 March 2020, EXCiPACT asbl has issued a new procedure for audit postponement and the use of remote audits of pharmaceutical excipients suppliers.
The procedure is published as an Annex to the 2017 edition of the “EXCiPACT Certification Standards for Pharmaceutical Excipient Suppliers” and can be downloaded from the homepage of the website www.excipact.org.
It affects only those pharmaceutical excipient manufacturers and distributors who are already EXCiPACT certified. Re-certification or surveillance audits may be impossible due to the strict travel ban and safety rules in place. These restrictions affect both Certification Body auditors and staff from excipient manufacturers and distributors to help them retain or renew their certification status.
The procedure describes alternative options for the Certification of Pharmaceutical Excipient manufacturers and distributors. It indicates the conditions and time-periods which are acceptable to postpone a re-certification or surveillance audit or to replace it by a remote audit. It has been communicated to each EXCiPACT Registered Certification Body for implementation as part of their internal procedures.
These new audit requirements remain valid while the COVID-19 pandemic containment measures are in place in the affected world regions and until local regulation travel restrictions are suspended, when physical (on-site) audits may return.
EXCiPACT Certification audits (i.e. those required for a first certificate) are out-of-scope of this procedure.
The procedure is available via this link. For further information, please contact the EXCiPACT Secretariat.
Extended deadline for risk assessment of nitrosamine impurities
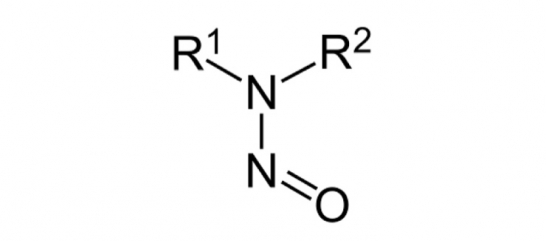
The European Medicines Agency announced the extension of the deadline to submit these risk assessments (step 1) of the review of manufacturing processes to 1 October 2020 in light of the challenges and impact of restrictions related to the current COVID-19 pandemic.
Late in 2019, IPEC Europe prepared a questionnaire to facilitate gathering information on pharmaceutical excipients for drug product manufacturers, available here.
Marketing Authorisation Holders are encouraged to submit the outcome of risk evaluations (step 1) whenever results are available and report back to the agency by using the appropriate template where a risk is identified or is not identified.
Further details of all requirements can be found here.
Information on nitrosamines for marketing authorisation holders - Questions and answers
The European Medicines Agency and the Co-ordination group for Mutual recognition and Decentralised procedures – human (CMDh) also updated the questions-and-answers document for marketing authorisation holders who are currently reviewing their medicines for the possible presence of nitrosamines and testing products at risk.
The document, now on Revision 3, includes the following updated questions and answers:
- Q4: How should tests be conducted – development of analytical methods for the determination of nitrosamines
- Q5: When should MAHs report to competent authorities – how to submit risk assessments results
- Q6: Interim limits for nitrosamines for non-sartan products
- Q13: Approach for new and ongoing marketing authorisation applications
The updated document is available here.
Regulatory News: Europe
European Commission & European Medicines Agency
COVID-19: What’s new
The European Medicines Agency provides timely updates on all the latest news and press releases on the COVID-19 pandemic in the COVID-19: What’s New page.
European Medicines Agency
Availability of medicines during COVID-19 pandemic
EMA and national authorities are putting measures in place to help prevent and mitigate possible disruptions to the supply of medicines. An Executive Steering Group on shortages of medicines caused by major events was organised in early March and meets weekly.
European Medicines Agency
E171 – Titanium Dioxide: updates
The European Commission published an update on titanium dioxide in food products. DG SANTE proposes to amend the current applicable Annex to Regulation 231/2012 to reflect the latest technically achievable limits for impurities in E171. The proposal will be analysed by the Novel Food and Toxicological Safety section of the Plants, Animals, Food and Feed (PAFF) committee.
European Commission
Brexit: New Notice to Stakeholders
The European Commission (DG SANTE) and the European Medicines Agency published the third version of the Notice to Stakeholders “Withdrawal of the United Kingdom and EU Rules for medicinal Products for human Use and veterinary medicinal Products". The document, originally published in February 2019, includes many important aspects and clarifies the soon-to-be status quo when the transition period will end in December 2020. ECA Academy lists many of the important changes to consider.
European Commission
Targeted Public Consultation – Revision of annex 21 of EU GMP Guide: extended deadline
The Annex 21, titled,"Importation of medicinal Products", summarizes the GMP requirements applicable to a Manufacturing Import Authorisation (MIA) holder which imports medicinal products (human and veterinary) from third countries. The consultation phase will close on 20 August, three months later than the original date of 20 May (as mentioned in the previous edition of Excipients Insight).
European Commission
European Directorate for Quality of Medicines (EDQM)
EDQM and COVID-19:
The European Directorate for the Quality of Medicines in its efforts to ensure the quality of medicines during the COVID-19 pandemic is working hard to maintain the continuous supply of goods and services. It is engaging with all stakeholders, including national, European and international authorities, to help protect public health in this regard.
EDQM
Pharmeuropa 32.2 released: comments until 30 June
42 draft texts have been published in Pharmeuropa 32.2 for public consultation. The deadline for comments is 30 June 2020.
EDQM
UK Medicines and Healthcare products Regulatory Agency
Guidance on Coronavirus - GxP regulation temporarily relaxed
UK regulatory agency MHRA communicated that temporary flexibilities on the rules for good manufacturing practices (GMP) and good distribution practices (GDP) will be allowed to address the current exceptional circumstances. The agency relaxed in early April the GDP requirements for wholesalers and extended the discretionary scope for qualified persons (QPs).
Exceptional GMP flexibilities for medicines manufacturers during the coronavirus outbreak
Exceptional GDP flexibilities for medicines during the coronavirus outbreak
Exceptional GMP flexibilities for medicines imported from third countries during the coronavirus outbreak
Approval of GxP documents when working from home during the coronavirus (COVID-19) outbreak
MHRA Guidance on Coronavirus
Regulatory News: rest of world
U.S. Food and Drug Administration (FDA)
Webcast - Nitrosamine Impurities in Drugs: What Health Care Professionals Need to Know
The CDER (Center for Drug Evaluation and Research) hosted a webinar on 5 May that provided an overview of nitrosamine impurities identified in several prescription and over-the-counter drug products. The presentation included background information on nitrosamines, a discussion of pharmaceutical quality, FDA's role in protecting the public, and an overview of public outreach and engagement.
View the webinar here (Adobe Connect required) - Download the presentation slides
COVID-19-Related Guidance Documents for Industry, FDA Staff, and Other Stakeholders
The Food and Drug Administration provides recommendations, regulatory information, guidance, and technical assistance necessary to support rapid COVID-19 response efforts.
FDA
Manufacturing, Supply Chain, and Drug Inspections due to COVID-19
CDER published guidance to provide clarity on manufacturing and supply chain changes, including expediting changes as needed, and on inspections during the emergency.
FDA
CDER Generic Drugs Forum 2020 – Session recordings and slides
All presentations of the 2020 CDER Generic Drugs Forum, organised by FDA on 15-16 April, are now available.
This YouTube playlist contains all session recordings.
FDA – SBIA Education Events via IPEC-Americas
FDA Creates Coronavirus Treatment Acceleration Program (CTAP)
The FDA created at the end-of March a new program to expedite the development of potentially safe and effective life-saving treatments. The Coronavirus Treatment Acceleration Program (CTAP) is using every available method to bring new therapies to sick patients as quickly as possible, while at the same time supporting research to further evaluate whether these medical countermeasures are safe and effective for treating patients infected with this novel virus.
FDA
Temporary policy for the preparation of alcohol-based hand sanitizer products during the COVID-19 emergency
The Food and Drug Administration (FDA or Agency) is announcing the availability of a guidance for industry to communicate its policy on alcohol-based hand sanitizer products for the duration of the public health emergency.
FDA via IPEC-Americas
U.S. Pharmacopoeia (USP)
COVID-19: Strenghtening the global medicines supply chain
The U.S. Pharmacopoeia published “Key Elements to Building a More Resilient Supply Chain”, a position paper reflecting on the impact of disruptions on the quality and safety of medicines in the global supply chain.
USP / Position paper
International Council for Harmonisation (ICH)
ICH Q3C(R8) with three new PDEs for comments
The ICH Q3C(R8) draft Guideline on Impurities: Guideline for Residual Solvents, has reached Step 2b and now enters the consulation period. The draft version contains only the PDE levels for three solvents. The public consultation is open until 30 July 2020.
ICH
International Coalition of Medicines Regulatory Authorities (ICMRA)
ICMRA aims for international alignment on policy approaches and regulatory flexibility during COVID-19 pandemic
Members of the ICMRA pledged to work together to speed the development and approval of medical products to contrast COVID-19. In the statement, regulatory agencies say that they will work together to align regulatory requirements and processes.
ICMRA
Calendar

All meetings are scheduled to take place online (TC) until further notice.
|
Group
|
2020
|
|
IPEC Europe Board
|
24 June, TC
23 September
25 November
|
|
Good Distribution Practices Committee
|
8 June, TC
1 July, tbc
29-30 September
1-2 December
|
|
Quality & Regulatory Affairs Committee
|
2 July
27 October
|
|
Multicompendial Compliance TF
|
19 May, TC
|
|
Lactose Interest Group TF
|
26 May, TC
22 September, TC
|
|
Co-processed excipients TF
New Task Force – kick-off teleconference
|
May, TC
|
Recommended readings

The Quality Lowdown: Coronavirus Prompts EU To Extend Comment Deadline For Annex 1
Pink Sheet included in its “The Quality Lowdown” column the latest IPEC Federation position paper on data integrity.
Pink Sheet ($)
NSF International announces updated GMP Standard for Pharmaceutical Excipients
The NSF/IPEC/ANSI 363-2019: Good Manufacturing Practices (GMP) for Pharmaceutical Excipients standard has been revised to align more closely with the structure of ISO9001:2015 (EN) Quality Management Systems — Requirements.
NSF via IPEC-Americas
Events

Here is a round-up of forthcoming events of interest to suppliers and users of excipients. Please let the IPEC Europe Secretariat know if we've missed one.
EXCiPACT Webinar : Pharmaceutical Excipients: The Need for Remote Audits in the Time of COVID-19
Webinar - 22 May 2020, 16:00
More information here
2020 PDA Annual Meeting – NEW DATES
Virtual conference – 20-22 July 2020
More information here
12th conference of the European Paediatric Formulation Initiative (EUPFI)
Virtual conference – 9-10 September 2020
More information here
2020 ISPE Europe Annual Conference – NEW DATES
Virtual conference – 16-17 September 2020
More information here
IPEC Europe Excipient Conference
Frankfurt, Germany – 17-18 September 2020
More information here ¦ REGISTER NOW
Making Pharmaceuticals Ireland
Dublin, Ireland – 29-30 September 2020
More information here
POWTECH 2020
Nürnberg, Germany – 29 September – 1 October 2020
More information here
CPhI Worldwide 2020
Milan, Italy – 13-15 October 2020
More information here
Making Pharmaceuticals UK – NEW DATES
Coventry, United Kingdom – 26-27 October 2020
More information here
23rd APIC/CEFIC Global GMP & Regulatory API Conference
Amsterdam, The Netherlands – 28-30 October 2020
More information here
ChemSpec Europe – NEW DATES
Cologne, Germany – 11-12 November 2020
More information here
|