Editorial

Dear Members,
Welcome to the latest edition of Excipients Insight. While everybody is now preparing for the summer break, the Secretariat remains busy and engaged in preparing for our events and meetings in the second part of the year.
Firstly, the 2021 IPEC Europe Excipient Conference will be held online on the afternoons of 22 and 23 September, with the “hot topics” in the area of excipient regulation and technology. The regulatory sessions will cover regulation in Europe and emerging countries, news from EDQM and USP as well as a look into the role of excipients out to the year 2025 and beyond. Presentations on formulation of medicines, 3D printing and continuous manufacturing also feature in the programme; the upcoming edition of Excipients Insight will report on the event.
Meanwhile, don’t forget to book your spot here – registrations before 31 July benefit from the reduced early bird fee.
Another date not to be missed is the quarterly catch-up on IPEC Europe activities, scheduled for 30 September. These one-hour meetings reserved for Members are very much in line with IPEC Europe’s direction of greater transparency, sharing information with Members in an interactive medium, closer to when things are happening.
Continuing in this vein, the Board also decided to organise the Forum 2022 in March, later than in previous years, to hopefully be able to organise it as a live event.
We organise these meetings to deliver on our mission's promise to inform and exchange good practices, to help you our Members meet regulatory expectations in the most efficient manner. Please give us your feedback on how well we’re doing... it's very much appreciated.
On the regulatory front, the headline news at the moment is the EFSA’s opinion on E171 Titanium Dioxide, no longer considered safe as a food additive. The impact on the pharmaceutical sector and the potential proposed ban shows how important it is to work and dialogue with regulators and other stakeholders. IPEC Europe has shared its perspectives on the potential loss of E171 on accessibility and availability of medicines with EMA and MHRA, and engaged with a E171 task force including EFPIA, MFE, and AESGP in the last months. This very dynamic situation is being closely monitored and we’ll provide more information on developments as they become available.
So, enjoy reading this latest edition of Excipients Insight, and enjoy your summer vacation.
Frithjof Holtz
IPEC Europe Chair
Catch-up with IPEC Europe activities

The IPEC Europe Board developed a series of quarterly virtual catch-ups to provide Members with timely updates on the association’s projects, giving the opportunity to interact with the Board and Committee/Task Forces leaders in a relaxed atmosphere, and raise possible topics to be added to the IPEC Europe work programme.
A dedicated group within the IPEC Europe Board, the Knowledge Sharing group, focussed on the structure of these meetings and after the first event on 17 March, the positive feedback and the interest generated made this virtual meeting a rendez-vous not to be missed!
Usual topics discussed include the review of a selected committee/task force’s activity, an update on the Project Tracker and current key priority projects, and what’s going on in the IPEC Federation and EXCiPACT. A recording of the latest virtual catch-up, organised in mid-June, is available here; previous broadcasts are available in the Member’s Lab, under Members / Catch-up with Members .
The next programme is scheduled to take place on 30 September – please keep an eye on our website for the Agenda, and don’t hesitate to contact the Secretariat with any questions and ideas.
The meetings are reserved to current IPEC Europe Members.
Project Tracker: monitoring IPEC Europe’s endeavours!
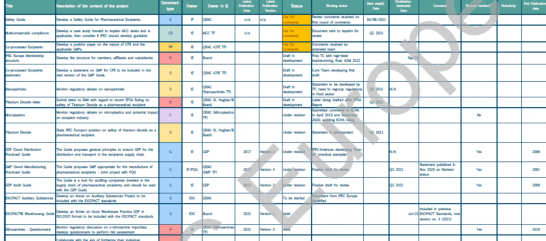
Previewed during the ‘launch’ catch-up with IPEC Europe Members on 17 March, the Project Tracker is a useful document accessible by all Members to review ongoing activities in the association. The Project Tracker, updated regularly, lists all the major projects on the current workplan and aims to provide Members with information on the status of Guides, position papers, and a sneak peek on completed projects. Of course, the document and featured key priorities are presented at quarterly catch-ups (see dedicated article).
The Project Tracker is reserved for Members only and is available in the Member’s Lab, under Members. Access to the Member’s Lab can be requested from the Secretariat.
IPEC Europe Annual General Meeting 2021

The IPEC Europe Annual General Meeting (AGM) took place virtually for the first time on Wednesday 9 June.
More than 60 attendees joined this important event to hear more on the main activities of the association. Unlike previous years, the AGM focused on mandatory legal matters; committees and task forces will provide updates on onjectives and progress in the Catch-ups with Members (see dedicated article).
First, IPEC Europe Chair Frithjof Holtz (Merck KGaA) presented a 2020 highlights and objectives for 2021, defining the priorities and the goals of the association for the upcoming months, while Karsten Diehl (BASF), the Treasurer, presented the financial status of IPEC Europe.
Mahmud Yunis (BIOGRUND, Producers group) was re-elected to the Board for a three-year term of office. The profile of all Board members is available on the IPEC Europe website.
Presentations made by Board officials are available in the Member’s Lab: Members / Annual General Meeting / 2021 Annual General Meeting. The Finances presentation as well as a recording of the AGM are available on request to the Secretariat.
Novel excipients: joint actions with EFCG

Novel excipients are essential for the development of new medicines and vaccines. They foster patient access to innovative and affordable medicines. Their use can also help to formulate drugs improving their efficacy, quality and safety.
There is currently a lack of regulatory pathway for the independent approval of novel excipients in Europe, which is a strong hindrance to innovation in the development of medicines. This is highlighted by delays in the approval of COVID-19 vaccines in the EU.
IPEC Europe and EFCG continue to make the case for Novel Excipients in Europe; a joint position paper illustrates the initiatives proposed to reduce barriers to innovation and complement the Pharmaceutical Strategy for Europe, to increase EU citizens’ access to high-quality and safe medicines as well as increase competitiveness towards other global markets (such as China and USA). Additionally, an article appeared on CPhI shows how the COVID-19 pandemic has led to a significant increase in the manufacturing of pharmaceutical products and vaccines, thus increasing the use and importance of excipients.
IPEC Europe and EFCG propose a set of measures to adapt the EU regulatory framework for the development of high-quality, effective, and safe medicines to enable this:
- A modular approach such as the Master File System for APIs (ASMF) should be extended to novel excipients providing independent review. Data needs for novel excipients are equivalent to those for APIs and regulatory equality is essential.
- Cross-referencing a novel excipient Master File in a Marketing Authorisation Application (MAA) would ensure full review by EMA, helping to shorten approval times and simplify authorisation procedures.
- Novel excipient manufacturers could work in parallel with developers of medicines again reducing approval times, especially in crisis situations.
- The confidential manufacturing know-how of the novel excipient will be protected.
IPEC Europe and EFCG continue to pursue their support for the creation of a regulatory pathway for the independent approval of novel excipients in Europe, to foster innovation in the development of medicines.
QRAC Committee

In the three meetings held in the first part of the year, the QRAC Core Team made substantial progress on a number of key objectives such as contribution to IPEC Federation Guides project, development of a substantive webinar programme, and initiatives on master files systems and regulatory issues.
On the EMF System, a position paper has been developed with EFCG and shared with the European Commission, also in the context of the feedback provided to DG SANTE for the Initiative on the Accessibility to Medicines.
A compilation of known global positions on nanoparticles has been shared with members for input (by the end of July), after which it will be approved and shared with IPEC Federation members.
After the publication of the Lactose position paper in February, the Interest Group’s efforts to develop an improved ?/β ratio method continue along with the usual monitoring activities.
A document on the ‘best practice’ to manage compliance with multiple compendial requirements has been drafted and has been shared with experts in the Committee for comments. Pending some further input, it will be routed for approval in the coming weeks. Following ECHA’s opinion on Restriction of intentionally added Microplastics, the dedicated task force has drafted a comprehensive ‘aide memoire’ as to how members can be informed of the requirements and make ready for compliance. The “How-to” document is being developed with IPEC-Americas for forthcoming publication.
The GMP Guide revision team is on a steady path to complete the alignment of the existing content with the most recent ISO 9001 standard on Quality Management (2015). The forthcoming edition of the guide will also include a statement on GMP status of CPEs (co-processed excipients) and mixed excipients. Considerable efforts are being made to ensure that the revision is finalised by the Core and Extended Revision teams in Autumn, for IPEC members to provide comments in the final months of 2021. The guide is planned to be published in the first quarter of 2022.
In addition to these ongoing activities, the team is considering to broaden the scope of IE activities to encompass raw materials in medical devices, and providing input to the development of the EXCiPACT Auxiliary Materials Annex.
Highlights of the QRAC Core meetings are available to all Members in the Member’s Lab, under Members / Committee - QRAC - Minutes / 2021 – Highlights.
The Chair of the QRAC Committee Johanna Eisele presented the objectives and the current activities of the group during the catch-up on 17 June (see dedicated article). Click here to watch her presentation, and contact the IPEC Europe Secretariat if you wish to join the Group.
The next meeting of the QRAC Committee will be held online in mid-September (16/9).
Titanium Dioxide - Updates

The long-awaited final EFSA opinion on E171 Titanium Dioxide concluded that it could no longer be considered safe as a food additive. The impact on the pharmaceutical sector is being considered by EMA via a task force including EFPIA, MFE and AESGP. IPEC Europe have engaged with this group in the last months and presented IE’s perspectives on accessibility and availability of medicines. IPEC Europe has also provided its position to EMA and MHRA in two separate letters.
Iron Oxides (E172) and Indigo Carmine (E132) are going through similar reviews; an EFSA opinion is expected within the 2022/2023 timeframe.
GDP Committee

The GDP Committee met three times during the first half of the year in January, March and May.
The Committee has been concentrating much of its efforts on revising the GDP Audit Guide, which will be published in September and presented by the Committee Chair Frank Milek at the IPEC Europe Excipient Conference in mid-September (see dedicated article).
A kick-off meeting with IPEC-Americas to include ‘How-to’s’ in the Good Distribution Practices guide took place in May.
Other activites from the Committee included the review and comments to the EXCiPACT Good Warehousing Practices Standard, contribution to the IPEC Glossary providing definitions of GDP and Distributor , and input to the Spanish translation of the IPEC Federation GDP Guide published in cooperation with SAYFBI.
The project on “Transport conditions for excipients” is currently postponed and will be resumed after the pandemic, when physical meetings will be again possible.
To volunteer to this group, please contact the IPEC Europe Secretariat.
Nitrosamine Questionnaire - new version and webinar

IPEC Europe published its revised “Questionnaire for Excipient Nitrosamines Risk Evaluation” (version 3) in March 2021. The questionnaire helps excipient manufacturers to support Nitrosamines drug product assessments, and has been praised and well received by the much of the excipients community, with more than 10000 downloads overall.
The third version of the Questionnaire includes 2020 regulatory updates, referencing the EMA assessment report “Nitrosamine impurities in human medicinal products” and related “Q&As”, as well as US FDA Guidance for Industry “Control of Nitrosamine Impurities in Human Drugs”.
New features are a matrix to help filter which sections need to be completed based on the structure and the origin of the excipient, and an optional conclusion.
A comparison Questionnaire, available here in PDF format, highlights the changes made in the new version. Click here to download the Questionnaire.
The leader of the Task Force, Dr. Ulrich Reichert (Merck KGaA), presented in a webinar attended by 300 participants the structure of the Questionnaire and the main updates included in the latest version.
The webcast is accessible here, while the slide deck can be accessed on the Member’s Lab in the dedicated folder Members / Events and Webinars / 2021.
Work is beginning on an IPEC Federation position paper on this topic.
IPEC Federation: Annual General Assembly & Strategic Focus

Annual General Assembly
The IPEC Federation organised its Annual General Assembly online on 26 April.
Delegates from all IPECs reunited virtually to discuss the results of the past year and 2021 objectives.
2020 was a constructive year for the Federation, which steered the publication of two position papers on Data Integrity and Good Manufacturing Practices Documents as well as two new guides on GMP Certification Scheme & Certification Body Qualification, and Quality by Design.
An ambitious revision programme resulted in publishing the updated Qualification Guide, the Excipient Information Package and Composition Guides.
The Federation submitted comments on several draft regulations across the world as well as fulfilling its observer role at the ICH.
In 2021, the Federation is pursuing its aggressive agenda to publish several new and revised guides which feature in the 2021 Strategic Plan. Good news is that so far this year, the new Validation Guide and the updated Glossary have been released, with the GDP Audit Guide planned for publication in the near future., A very exciting development which is in the preliminary stages of exploration is the creation of a database of global excipient requirements.
Strategic Focus and Priority Objectives for 2021
Built around the Federation’s area strategic focus, the Board has identified several high priority objectives for 2021: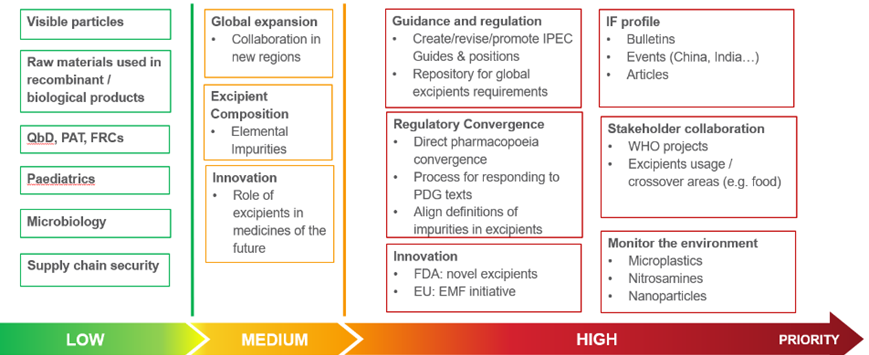
- Guidance and Regulation: Focus on the revision and creation of IPEC Guides; development of a new repository for global requirements;
- Innovation: Novel excipients and EMF;
- IF Profile: Bulletins, articles, events;
- Stakeholder collaboration: Contribute to WHO projects, cooperate on excipient use in crossover areas (e.g. food)
- Regulatory Convergence: Direct pharmacopoeial convergence, alignment of definitions of impurities in excipients, process to respond to PDG texts;
- Monitor the environment, with particular attention to Microplastics, Nitrosamines and Nanoparticles
At its monthly virtual meetings, the Federation members monitor the low and medium priorities to see if activity is growing in any of these areas, and action is needed.
IPEC Federation Connect
The new issue of IPEC Federation Connect is available on the Federation website. In addition to the articles above, you will find news from other IPECs, including and update on ICH Q13 , Risk Assessment in Japan, and the 30th Anniversary of IPEC-Americas.
IPEC Europe Excipient Conference 2021 : register now !

IPEC Europe and APV are delighted to invite you to our 9th annual conference on pharmaceutical excipients. For the second consecutive year, the conference will be held as a virtual event on the afternoons of the 22 and 23 September 2021.
As in previous years, the conference focuses on current “hot topics” in the field of pharmaceutical excipient regulation and technology.
The regulatory session will highlight current topics such as regulation in Europe and emerging countries, news from EDQM and USP as well as an outlook into the role of excipients to the year 2025 and beyond. The role of excipients in the development of intraoral and topical drug delivery systems. will also be featured.
Continuous manufacturing presents a flexible approach to oral solid dosage form production and meets the industry’s demands for faster product development, reduced costs and increased manufacturing flexibility. We will learn the suitability and specific requirements of excipients for this new manufacturing process. Compendial specifications may not address all the physiochemical properties that can have an effect on manufacturability and final product quality.
The versatility and flexibility of 3D printing technique offers a large spectrum of advantages and will likely implement substantially improved medications in near future. Here, participants will listen to a presentation of modified release tramadol printlets (3D printed tablets) with alcohol-resistant and abuse-deterrent properties prepared by direct powder extrusion three-dimensional (3D) printing.
The conference brochure is now available for download.
Benefit from the early bird rate until 31 July - to register, please click here.
The Conference offers you the opportunity to present your company, products and services to a truly focused target market: the sponsorship package offers you one virtual stand and one full conference registration for 990 € (VAT excl.). For detailed information about the sponsoring options, please contact Katrin Kälkert, kk@apv-mainz.de .
Maybi and Aenova Group join IPEC Europe

IPEC Europe is delighted to welcome Maybi and Aenova Group as the latest new members of the association.
MAYBI
Founded as a family company in Malkara in 1993, Maybi (the brand name of “Malkara Birlik Sur ve Sut Mamulleri A.S.) is the only lactose excipient manufacturer in Turkey and is the market leader of whey products in the country.
Maybi's range of products includes sweet whey powder, whey permeate, lactose, and pharma lactose. The company works in partnership with customers all over the world, exporting to more than 40 countries.
Learn more about Maybi on maybi.com.tr.
AENOVA GROUP
The Aenova Group is a leading global contract manufacturer and development (CDMO) services provider for the pharmaceutical and healthcare industry. As a reliable, long-term partner to pharmaceutical and consumer health care customers around the world, both in the human and veterinary healthcare market, Aenova Group’s services include end-to-end manufacturing and development of all dosage forms and potency levels (ranging from nutraceuticals to high-potents) out of 15 sites in Europe and the US.
Further information on Aenova can be retrieved on their website aenova-group.com.
IPEC Europe Forum 2022 - 30 years of IPEC Europe
IPEC Europe will celebrate its 30th anniversary years in 2022, and for this special occasion, the Board of Directors has agreed to organise the Annual Forum in the second part of March, later than in previous editions, to maximise the chances of hosting a live event.
The Forum is scheduled to be the first in-person IPEC Europe event since the 2020 Forum in Aix-en-Provence, and promises to be a landmark event for our association with many opportunities to celebrate this important milestone. The Annual General Meeting will be held prior to the Forum, on Wednesday afternoon, and will focus on legal and administrative matters. The Friday morning slot will be secured for the first catch-up with Members of 2022.
Further details on location and format will be shared in the autumn, while registration for the Annual Forum – which, along with the usual regulatory and scientific programme will in 2022 delve into the future of the EU regulatory environment - will open by the end of the year.
Keep an eye out for the programme and registration details, which will appear on the IPEC Europe website and in a later edition of Excipients Insight.
EDQM: new Director - call for volunteers on Calcium Acetate

The Council of Europe has appointed Petra Dörr, PhD, as future Director of the European Directorate for the Quality of Medicines & HealthCare (EDQM). She will take over the function from the current Director, Dr. Susanne Keitel, in October 2021.
Petra Dörr comes to the EDQM with more than 25 years of international experience in the public and private sector – in the pharmaceutical industry, with a regulatory authority and more recently with the World Health Organization (WHO).
Meanwhile, at its 170th session the European Pharmacopoeia (Ph. Eur.) adopted 69 texts that will be published in Ph. Eur. Supplement 10.8.
Three new monographs are included:
- Chinese motherwort (Leonuri herba) (2785)
- Purple coneflower herb expressed juice, stabilised with ethanol (2282)
- Purple coneflower expressed juice, stabilised without ethanol (2894)
The list of all adopted texts is available on the Ph. Eur. Work Programme page; the texts will become effective on 1 July 2022.
Calcium Acetate: call for volunteers
Calcium Acetate: call for volunteers
The latest issue of Pharmeuropa 33.3 contains 47 drafts published for review and comments. The full list is available here. The deadline for comments is 30 September 2021.
In this issue, a revised monograph for Calcium acetate includes the removal of the limit tests for chlorides, nitrates, and sulfates replaced with a unique ion chromatography method.
IPEC Europe is looking for volunteers to review the draft monograph and provide comments to EDQM. The comments must be submitted to EDQM by 30 September.
If you wish to join the task force, please inform the Secretariat no later than 20 August.
Making Pharmaceuticals UK is back!

On 5-6 October 2021, Making Pharmaceuticals is back at the Coventry Building Society Arena (formerly Ricoh Arena) in Coventry, UK. Making Pharmaceuticals will host over 2,000 visiting pharma professionals and more than 200+ exhibitors in six exhibition zones.
IPEC Europe will host a conference session on 6 October from 9:15am showcasing three presentations:
- IPEC Co-Processed Excipient Guide for Pharmaceutical Excipients– Adrian Bone, IPEC Europe Senior Advisor
- Best Practices for the Selection of Excipients for Paediatrics – Workshop Reflection – Kevin Hughes, Colorcon Ltd / IPEC Europe Board member
- TBA – Dr. Iain Moore, CRODA / EXCiPACT President
IPEC Europe/EXCiPACT will be jointly represented in the exhibition space – make sure to pay a visit to Stand 108.
Register for free to the Making Pharma Expo and Conference on the Making Pharmaceuticals website.
EuPFI Conference

The 13th conference of the European Paediatric Formulations Initiative (EuPFI) - a group which strives towards the development of better medications and dosage forms for children - will take place on 22-23 September online.
The conference will feature a dedicated session on the selection of excipients for paediatrics on day two, and will present on the excipient screening platform and the risk assessment of paediatric excipients. Other topics on the agenda include minitablet development/acceptability and COVID in paediatrics.
Additional information on the conference can be found on the EuPFI website.
Calendar

All meetings are scheduled to take place online (TC) until further notice.
|
Group
|
2021
|
| Catch-up on IPEC Activities - Q3 |
30 September |
|
IPEC Europe Board
|
21 September
24 November
|
|
Good Distribution Practices Committee
|
September
|
|
Quality & Regulatory Affairs Committee
|
16 September
16 December
|
Events

Here’s a round-up of forthcoming events of interest to suppliers and users of excipients. Please let the IPEC Europe Secretariat know if we've missed anything!
Q3 2021
IPEC Europe Excipient Conference
Online event – 22-23 September 2021
More information here / Register now
13th EUPFI Conference
Online event – 22-23 September 2021
More information here
Excipient World 2021
National Harbor, MD, USA – 27-29 September 2021
More information here
Q4 2021
Making Pharmaceuticals – Exhibition and Conference
Coventry, United Kingdom – 5-6 October 2021
More information here
APIC/CEFIC Global GMP & Regulatory API Conference
Berlin, Germany / live online - 26-28 October 2021
More information here
2020 ISPE Annual Meeting & Expo
Boston, MA, USA – 1-3 November 2021
More information here
CPhI Worldwide
Milan, Italy – 9-11 November 2021
More information here
2022
IPEC Europe Annual Excipients Forum
Location to be determined – late March 2022
Chemspec Europe
Frankfurt, Germany – 31 May – 1 June 2022
More information here
|