Editorial

Dear Members,
Seasons’ greetings and a warm welcome to the last edition of Excipients Insight for 2021.
No doubt you’re all busy with end-of-year activities and preparing for the new year! As I reflect on the editorial issued one year ago, we were very much hoping 2021 would see something of a return to our more normal lives. Well, thanks to aggressive vaccination campaigns, we took some steps to that goal but we’re not quite there yet. But let’s remain optimistic for 2022! We know that some of those vaccines contained excipients vital for their successful delivery which fits nicely with some of our efforts to promote wider uses of novel excipients.
If you were able to connect to this month’s catch-up, you’ll have heard that our task forces are very active, working on several initiatives which will carry over into the coming years. Some of these topics are new to us and requires us to reach out for expertise in non-traditional areas for IPEC Europe such as microplastics and sustainability. And here comes an appeal … if these subjects don’t fall within the responsibilities of your role, please connect with your colleagues who might be able to help strengthen our teams to help our members understand and position these new requirements from an excipient perspective, into their operations.
Nanoparticles, co-processed excipients and nitrosamines issues will continue to demand attention and I strongly believe our work on all of these subjects add great value to our members and our reputation as an association. While a full report of achievements and new objectives will be forthcoming, I would like to highlight now what a great year it’s been for IPEC publications. The new IPEC safety guide fills a much-needed gap in the excipient community and the work that’s been done on the IPEC PQG GMP guide really takes it to another level. Watch out for its release early in 2022.
So, as the year and indeed this editorial draw to a close, it remains for me to thank you for being part of IPEC Europe, especially to those contributing in committees and task forces, and continuing to make the association a success. And long may it continue as we move into our 30th year! We’ll share more details of how we will mark this occasion once the situation related to the pandemic is clearer. So, meanwhile, I wish you a healthy and prosperous 2022 and hopefully, we will be able to meet again in person in the not-too-distant future!
Frithjof Holtz
IPEC Europe Chair
Updates from IPEC Europe Board and GDP Committee

IPEC Europe Board
The IPEC Europe Board met virtually on 25 November to discuss achievements in different areas and brainstorm future objectives for 2022. The Board discussed the preliminary results of the membership survey and invited members to provide additional inputs (see dedicated articles). Proposals for a redesigned membership fee structure are being developed to attract new members and be more inclusive of small companies.
Good Distribution Practices (GDP) Committee
The GDP Committee met on 26-27 October in Stuttgart, Germany to review the activities of the committee and plan ahead for the new year.
The GDP Audit Guide was published on 14 September and a webinar took place on 7 December (see dedicated article); members also provided comments on the draft USP GC <1083> “Supplier Qualification” (see dedicated article). The team will contribute to the GDP “How-to” guide once IPEC-Americas develops the project charter. The team will review the content of the IPEC GDP Guide, now 5 years old, and will include transport of excipients and data integrity principles. The committee will reconvene in late March (date to be confirmed).
IPEC Europe Task Forces, activities aplenty!

Lactose Interest Group (LIG)
Since the publication of the IPEC position paper on the low risk of the use of Pharmaceutical Lactose in oral preparations (see related article on Guides and Position Papers from IPEC Federation), the group has been working on evaluating the reliability of the alpha/beta assay method. Also, in the ongoing dialogue with USP, the team provided the requested feedback on the optical rotation method in the framework of the revision of the USP-NF lactose monograph. The impact of new China decrees 248 and 249 on lactose products is being assessed.
Co-Processed Excipients (CPE)
The Task Force is working to establish a clear definition for CPE as excipients and clarify the GMP status of CPEs with regulatory authorities. Input has been provided to the GMP Guide revision team for the insertion of a statement on co-processed excipients in the forthcoming revised Guide. A letter and a presentation are being finalised to then engage with regulators and other industry associations to clarify the legal status of CPEs. An additional objective would be to revise the IPEC CPE guide.
Microplastics
This joint IPEC Europe and IPEC-Americas Task Force is in the process of completing a ‘how-to’ document which would complement available information on the ECHA Ruling on intentionally-added microplastics; the draft will be circulated in the QRAC Committee and Board in early 2022. The Team will focus then on the collection and screening of data on how excipients contribute to microplastics and reporting.
Nitrosamines
After the publication of the latest version of the Questionnaire in March, the Task Force is developing a position paper within the Federation on the risks excipients could present in generating nitrosamines in drug products. Members held a teleconference with IPEC-Americas during the autumn to review the comments of the first draft document.
Nanoparticles
The Team developed a compilation of existing legislation and guidance on nanoparticles, available to all Members in the Member’s Lab. Then a call of interested members was circulated and then the first teleconference (15 October) was organised to identify the problem statement of nanos in pharmaceutical excipients. This initiative will be part of an IPEC Federation project identifying the challenges of nanoparticles in the different region to develop a global position. If you are interesting to contribute, please contact the IE Secretariat.
Titanium Dioxide E171
In late October, the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) endorsed the European Commission recommendation to remove titanium dioxide from the list of allowed food additives andwill be banned from the food additives list in the course of 2022. EMA has been requested to provide an updated risk assessment on the use of TiO2 (E171) in pharmaceutical products by 1 April 2024. A final decision will be taken by 2025.
No significant regulatory issues have beenraised on the use of Titanium Dioxide outside Europe thus far.
Multicompendial Compliance
The Best Practices document was published in November (see dedicated article) along with an aide-memoire to help describe the document. Volunteers are wanted to fuel the IPEC Federation initiative “Mutual Recognition of Compendial Compliance” – please contact the IPEC Europe Secretariat by 15 December.
IPEC meets PDG - New harmonised General Chapter on chromatography

On 1 December, the IPEC Federation (IPEC) met with the Pharmacopoeial Discussion Group (PDG) to discuss common areas of interest related to the harmonisation of excipient monographs and general chapters. This event took place virtually, hosted by EDQM and was attended by IPEC representatives from the Americas, Europe and Japan.
PDG announced recently that it is preparing a pilot to integrate additional world pharmacopoeias into its membership. This is a critical step in the PDG’s commitment to expanding recognition of harmonised pharmacopoeial standards with a view to achieving global convergence. The publication of the Harmonised General Chapter Chromatography (G-20) was also highlighted (see separate article).
The agenda included follow up on a list of ‘priority’ excipients and general methods submitted by IPEC to PDG at an earlier meeting. IPEC would like PDG to consider these candidates for inclusion on the PDG workplan. PDG had gone to great lengths to evaluate the feasibility of harmonising the identified materials and methods; the latter IPEC feels will have important impact globally, particularly with reference to PDG’s expansion plans. IPEC agreed to provide additional data to support the rationale for further analysis and therefore, for evaluations to be concluded. This discussion extended to proposals from IPEC that topics such as FRCs, impurities and co-processed excipients would benefit from dialogue at this platform on their further development, as priorities allow.
IPEC attendees agreed that the meeting was conducted in a positive and constructive manner and we look forward to further exchanges with PDG in 2022.
PDG signs-off on milestone harmonised general chapter on chromatography
The harmonised general chapter Chromatography was signed-off by the Pharmacopoeial Discussion Group (PDG), which brings together the European Pharmacopoeia (Ph. Eur.), Japanese Pharmacopoeia (JP) and the United States Pharmacopeia (USP), on 28 September 2021. The coordinating pharmacopoeia for this text was the Ph. Eur.
During a joint PDG–industry meeting in 2009, the PDG was encouraged to add harmonisation of the three regional chapters on chromatography to the PDG work programme. Although the chapters in question differed in content and format, it was considered feasible to develop a chapter describing core requirements applicable for TLC, HPLC and GC.
After discussion, it was agreed not to include more general (textbook type) descriptions of individual techniques as each of the PDG pharmacopoeias has its own approach, decided at regional level.
These harmonised requirements promote the development of individual monographs with a consistent approach and enhance understanding of basic requirements by users in all three regions.
The successful completion of the complex work demonstrates once again the commitment of the participating pharmacopoeias to harmonisation and the PDG – ultimately benefiting patients and industry.
Source: EDQM
Project Tracker: latest updates
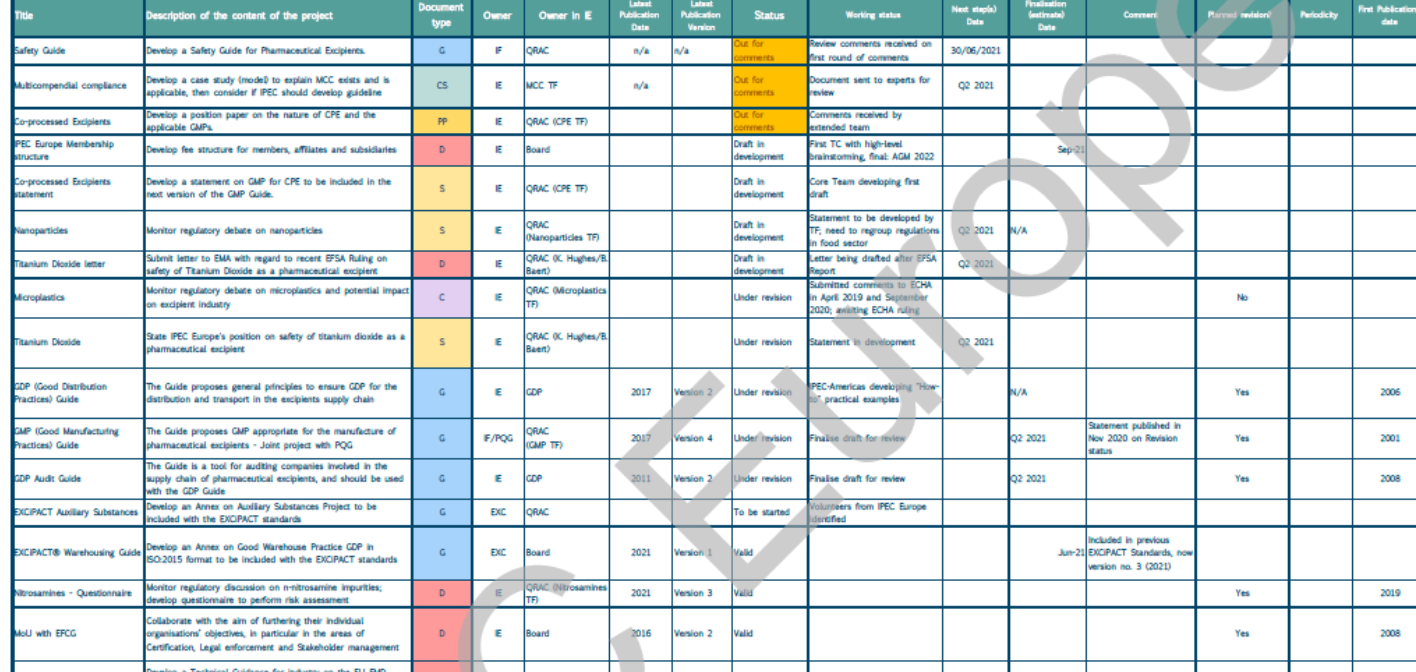
The Project Tracker is a document accessible by all Members to stay up-to-date on ongoing IPEC activities. The Project Tracker, updated regularly, lists all the major projects on the current workplan and aims to provide Members with the latest information on the status of Guides, position papers, and an overview on completed and ongoing projects.
Latest updates include:
- Microplastics ‘How-to’: document nearly completed, discussions ongoing with IPEC-Americas and IPEC Federation
- IPEC-PQG GMP Guide: the draft is being finalised and will be submitted for broader review in the first months of 2022 to all regional IPECs
- EXCiPACT Auxiliary Substances: the team is split into smaller groups to work on specific items
- Sustainability chapter of the EIP: the chapter is under development and should be finalised by end-2022
- Certificate of Analysis/Stability/Significant Change guides: Charter and Teams are being identified.
A shortened version of the Project Tracker with featured key priorities was presented at the Catch-up with Members – Q4 on 9 December (see dedicated article). Rewatch the session here.
If you wish to volunteer and join any of the groups/teams, please contact the Secretariat.
The Project Tracker is reserved for Members only and is available in the Member’s Lab, under Members. Access to the Member’s Lab can be requested from the Secretariat.
IPEC Europe Excipients Forum 2022

The eagerly awaited 2022 Forum will be an opportunity to celebrate IPEC Europe’s 30th Anniversary and the 10th Anniversary of EXCiPACT. The Forum was planned to take place as an in-person event in Lisbon, Portugal in late March.
The increased instability and the difficulty to predict how the pandemic situation will evolve in the coming weeks led the IPEC Europe Board to review the situation.
As a result, the Forum will not take in place in late March as previously announced but in a different setting in Q2 2022 – date to be announced - to maximise the chances of hosting a face-to-face event. We look forward to celebrating the anniversary of IPEC Europe and its many accomplishment with past and present Members alike.
Please keep a close eye on our website and LinkedIn page for more details.
IPEC Safety Guide

The International Pharmaceutical Excipients Council Federation, (IPEC Federation) announces the availability of a new IPEC Safety Guide for Pharmaceutical Excipients. This guide is applicable globally.
Building off articles published by both IPEC-Americas (A New Approach to the Safety Assessment of Pharmaceutical Excipients) and IPEC Europe (The Proposed Guidelines for the Safety Evaluation of New Excipients) in 1996 and 1997, respectively, the IPEC Safety Guide has been designed to give an overview on recommended toxicological studies for different therapeutic applications, routes of administration and treatment periods. Toxicological safety studies described in this guide are intended for consideration by excipient manufacturers who market excipients for use in drug formulations and excipient users who conduct toxicology studies required for the initial approval of a novel excipient in a drug formulation. Excipient users formulating an excipient beyond its approved, prior use are responsible for conducting the appropriate safety studies.
At the time of publication of this guide, with the exception of a pilot program for novel excipients review recently introduced by the US FDA, there is no independent review of novel excipients and this guide is intended to provide information to assist in determining the level of safety information necessary to support the use of a novel excipient, taking into account that depending on the type of novel excipient, different levels of safety information may be required.
The Guide includes several clinical therapeutic areas for novel excipients based on intended use in special patient populations when specific considerations are required to assess potential excipient safety concerns such as: pediatric safety, endocrine activity, nano character and biological occurrence. In addition, several recent New Approach Methods (NAMs) are discussed to introduce the global emergence of alternative in silico and in vitro methods to complement traditional in vivo approaches. These approaches are being used to employ a Weight-of-Evidence (WoE) approach to demonstrate the safety of novel excipients consistent with the growing regulatory commitment to reduce animal testing in safety assessments.
The IPEC Safety Guide is available on the Member’s Lab as an exclusive preview for IPEC Europe Members.
A webinar will be held in the forthcoming months – please keep an eye on our website.
Webinar – IPEC GDP Audit Guide

IPEC Europe held its final webinar of the year on 7 December, covering the principles of Good Distribution Practices and how the guide can be used to assess compliance and drive continuous improvement.
The online event, presented by Dr. Frank Milek, Chair of the GDP Committee within IPEC Europe, President of IPEC Federation and Head of GMP and SHEQ Operations for Aug. Hedinger, attracted more than 90 attendees. Frank provided an overview on Good Distribution Practices for starting materials, the importance of a secure supply chain and how these principles are reflected in the IPEC Excipient Good Manufacturing Practices guide, a tool to audit excipient supply chain. This insightful presentation triggered an interesting Q&A session moderated by IPEC Europe Senior Adviser Adrian Bone.
Review the presentation here: Webinar – IPEC GDP Audit Guide
IPEC Federation guides and papers of 2021

The collaborative efforts of the five IPECs helped publishing four IPEC Federation guides and one position paper in 2021. Here is a short summary of all guides and papers published by the IPEC Federation. All these documents are available in our website and/or Member’s Lab, reserved to IPEC Europe Members. If you do not have your credentials yet, please contact the Secretariat: info@ipec-europe.org
Position Papers
Pharmaceutical Lactose used in oral preparations is a low-risk excipient
Regulatory authorities worldwide increasingly seek to classify pharmaceutical excipients according to the risk they may present to patients when used in dosage forms.
Although lactose is derived from an animal derived material (whey), this position paper seeks to support pharmaceutical grade lactose as a low-risk excipient in oral dosage forms. IPEC Federation considers that pharmaceutical grade lactose has a low risk profile with respect to chemical and biological risks when used in oral pharmaceutical preparations.
Read the Position Paper here
Guidelines
IPEC Validation Guide
The guide was developed to help describe activities taken by excipient manufacturers to test and evaluate their systems, processes and methods in order to provide a high degree of assurance that they consistently produce results meeting predetermined acceptance criteria. It recognizes that an excipient manufacturer often does not perform “validation” activities in the same manner or to the same extent as the pharmaceutical industry, however, many activities that they do perform leads to the same degree of assurance. Read here the full press release.
Download the IPEC Validation Guide
IPEC Glossary of Terms and Acronyms
First published in 2014, the updated Glossary of Terms and Acronyms introduces a new set of criteria for terms/definitions to be included in future versions of the glossary. Read more about these criteria here. Another new feature to this Glossary is a column that includes reference to a list of external sources where the term has been defined. Several of the sources include ICH Guidelines and WHO Technical Reports where the definitions are often more applicable to API than excipient; therefore, the defined definition in this Glossary may have been modified to be more appropriate for an excipient.
Further, an Excel workbook containing mapping for terms and acronyms found in each of the current IPEC guides was developed and will be available to IPEC members. The Excel workbook also contains a list of ~700 pharmaceutical terms and definitions currently found and defined in external glossaries (e.g. ICH Guidelines, WHO Technical Reports, FDA Guidances and webpages).
Download the IPEC Glossary of Terms and Acronyms
Download the Excel spreadsheet (Member’s Lab)
IPEC Good Distribution Practices Audit Guide
The IPEC GDP Audit Guide provides a comprehensive tool for companies auditing the supply chain of pharmaceutical excipients. Several incidents in the past were caused by a lack of supply chain security and inappropriate handling of pharmaceutical excipients. This has moved regulators, users, manufacturers and distributors to take action.
The GDP Audit Guide should be used in conjunction with the IPEC Good Distribution Practices Guide. It serves as a valuable tool to help the auditor conduct a complete audit of all relevant GDP principles for pharmaceutical excipients. Read the full press release.
Download the IPEC GDP Audit Guide (Member’s Lab)
IPEC Safety Guide
See the dedicated article. Read here the full press release.
Download the IPEC Safety Guide (Member’s Lab)
Catch-up with IPEC Europe – Q4

The last catch-up of the year on IPEC Europe activities took place on 9 December with the usual updates on the current projects of the association.
After a brief review of the topics touched during the four catch-ups of 2021 and an overview of the current activities from the Project Tracker, Task Forces were on the spotlight, with Johanna Eisele, Christian Becker and Adrian Bone presenting the activities of the Co-processed Excipients, Microplastics, Nanoparticles and Multicompendial Compliance Task Forces. An update on the status of the revision of IPEC Guides was also provided. If you wish to be involved in these groups or receive more information, please contact the Secretariat.
Up next, Amina Faham provided an update on the Membership Survey and encouraged all Members to participate to this important survey to help the Board building the future of the association.
The recording of the virtual catch-up is available here; the slide deck and previous broadcasts are available in the Member’s Lab, under Members / Catch-up with Members .
The next catch-up is scheduled to take place in March – please keep an eye on our website for the registrations and the Agenda, and do not hesitate to contact the Secretariat with any questions and ideas.
The meetings are reserved to current IPEC Europe Members.
IPEC Europe Best Practices Document on Multicompendial Compliance Strategy

IPEC Europe is pleased to announce the availability of a ‘Best Practices’ document, "Executing a Multicompendial Compliance Strategy for Pharmaceutical Excipients".
This was developed with the goal of aligning procedures commonly used within companies, to achieve compliance with multiple compendia without performing multiple tests for given excipient attributes.
The approach proposes an eight-step process which can result in one pharmacopoeial test method being selected to represent other pharmacopoeias in scope, without compromising product quality and patient safety.
While similar approaches have been accepted by various stakeholders including regulators, regulations and regulatory guidance will always supersede any company’s effort to employ this process.
In addition to the document itself, IPEC Europe has summarised the methodology in a concise, easy-to-read infographic.
The Best Practices document and the infographic are available to download immediately via www.ipec-europe.org.
USP <1083> Supplier Qualification – IPEC Europe comments

In early September, USP published a proposed chapter on Supplier Qualification in Pharmacopoeial Forum 47(5) for comments.
This proposed chapter is aimed at discussing the importance of supplier qualification and the application of a quality risk-based approach to select, assess, approve, and monitor suppliers of materials and services. In this chapter, USP focused on the high-level elements of supplier qualification that apply to all organizations and outline a five-step process for supplier qualification.
IPEC Europe provided comments alongside IPEC-Americas and the other sisters associations on this proposed General Chapter, stating that Supplier Qualification would be best served with more specific industry or regulatory guidance rather than with this proposed GC.
The comments letter submitted to USP is available in the Member’s Lab, under Members / Submissions to public consultations.
ICH : November Assembly Meeting

ICH published the report of the latest Assembly Meeting in November, building on the many ongoing Global Harmonisation Efforts. Below an extract of the full report, available here.
Further expansion of ICH Membership and Observership
The ICH Assembly welcomed COFEPRIS, Mexico as a new ICH Member, in addition to three new ICH Observers: EDA, Egypt, Indonesian FDA, Indonesia and SECMOH, Ukraine, bringing ICH to a total of 19 Members and 35 Observers.
Progress on existing ICH Guidelines and harmonisation activities
ICH’s Working Groups have also continued to progress their activities virtually and the Assembly was updated on the status of ICH’s Working Groups. The Assembly noted significant milestones reached recently by several Working Groups including:
• Step 2 reached in November 2021 for the ICH Q9(R1) draft Guideline on Quality Risk Management (QRM) which is intended to provide important additional guidance on four specific areas: the levels of subjectivity in risk assessments and in QRM outputs; the product availability risks; the lack of understanding as to what constitutes formality in QRM work; and the lack of clarity on risk-based decision-making.
• Step 2 reached for the ICH Q13 draft Guideline on Continuous Manufacturing of Drug Substances and Drug Products in July 2021.
In addition, the Assembly approved a report from the Quality Discussion Group outlining future opportunities for ICH Quality Guidelines to support continual improvements and innovation in manufacturing technologies and approaches that would be subsequently published on the ICH website.
New topic selection process
The ICH Assembly decided to proceed with the New Topic selection process cycle in 2022. Considering the current COVID-19 influence and the delay with some existing topics, the Assembly will seek topic candidate(s) with high public health impact and in specific areas. The Assembly will also seek more efficient WG management to progress work on more ICH Guidelines.
Communication
The Assembly noted the ICH 30th Anniversary Publication published on the ICH website in October 2021, providing an overview of ICH’s history and current work, as well as views of different stakeholders on how ICH has contributed to better health and ICH’s future directions in the next 10 years. An informative leaflet on ICH will also be finalised shortly after the meeting.
EMA consultation: Veterinary concept papers

The European Medicines Agency has published for public consultation the following veterinary papers:
As IPEC Europe does not cover veterinary medicines routinely; this is shared for information purposes only.
Comments should be provided using this template and sent by the indicated deadline.
Calendar

|
Group
|
2022
|
|
IPEC Europe Board
|
17 January
|
|
Good Distribution Practices Committee
|
late March
|
|
Quality & Regulatory Affairs Committee
(Core Group)
|
16 December
|
| Co-processed Excipients TF |
TBA |
| Microplastics TF |
mid January |
| Lactose Interest Group |
TBA |
| Catch-up on IPEC Activities - Q4 |
late March |
All meetings will be held online unless mentioned otherwise.
Do you wish to join a Committee or a Task Force? Please contact the IPEC Europe Secretariat.
Events

Here’s a round-up of forthcoming events of interest to suppliers and users of excipients.
For a more comprehensive list, please click here (source: R Chaudrey)
Please let the IPEC Europe Secretariat know if we've missed anything!
Q1 2022
IPEC Europe Excipients Forum 2022
More information soon
Exciperience
Online event - 9-10 March 2022
More information here
PBP World Meeting
Rotterdam, The Netherlands – 28-31 March 2022
More information here
Making Pharmaceuticals UK
Coventry, United Kingdom – 26-27 April 2022
More information here
Chemspec Europe
Frankfurt, Germany – 31 May – 1 June 2022
More information here
POWTECH 2022
Nuremberg, Germany - 30 August - 1 September 2022
More information here
IPEC Europe Excipient Conference 2022
Frankfurt, Germany – 27-28 September 2022
More information soon
|